As
reported in today's Olympia (Wash.)
Olympian, the FDA's Pediatric Ethics subcommittee will
meet on Sept. 10 to discuss whether "[i]t is ethical in the name of science to give a healthy child as young as 9 a controlled substance." The story continues:
The research, proposed by the National Institute of Mental Health, includes healthy children among 9- to 18-year-olds who would receive a single 10 mg. dose of dextroamphetamine. The hoped-for payoff for research: A better understanding of how healthy brains work differently from those of children diagnosed with attention deficit hyperactivity disorder.
The payoff for families: $570.
The study would look at the effects of dextroamphetamine (the active ingredient in Dexedrine and Adderall) . It is similar to a study (by Judith L. Rapoport, chief of child psychology at the National Iinstitute of Mental Health) 20 years ago, which found increases in attention span regardless of whether the child-subject had ADHD. The new study would be similar, but it would add an MRI to map brain responses to the drug. Despite the similarities between the two studies,
review boards that balance risk vs. scientific gain have changed dramatically in 20 years. Indeed, an NIH review panel met twice and was unable to reach a consensus whether risk to healthy volunteers would be too high in the new study. They kicked the sensitive matter over the FDA's new pediatrics ethics subcommittee.
The scientific merit of the study -- which will include 14 children with ADHD, 14 healthy children, 12 pairs of identical twins, and 12 pairs of fraternal twins -- was confirmed by a NIMH panel last year. But a safety panel was concerned about exposing healthy children to a Class 2 controlled substance, the potential for future drug abuse after the experimental exposures, and the possibly coercive effect of a $570 payment to each participant in the 11-hour study. From past studies, we also know "[t]he most common side effects among healthy children given a single dose of the stimulant in past experiments was temporary insomnia and poor appetite. One brain-damaged child exposed to the medication suffered hallucinations."
So where would your average institutional review board come down on the question if presented with this research protocol?
Many ethicists expect the FDA subcommittee to use a primary litmus test: Does taking the stimulant pose more than a minimal increase to risks that healthy children face in everyday life?
Pearl O'Rourke, who oversees human research affairs, interviewed the heads of six review boards at Massachusetts General and Brigham and Women's Hospital.
"Five said they would not approve this study. And all five said, 'But we wish we could,"' O'Rourke said during a March 3 NIH discussion.
O'Rourke acknowledged that the review boards struggle with murky federal regulations, tightening case law, financing agencies that prefer pediatric studies and the threat of negative media coverage.
"I live in dread fear of what's going to be on the front page of the paper," she told the audience. "So, when I heard this, three things hit my mind: Kids, ohmigod! Psychiatric disease. And a class 2 drug."
The impact of federal regulations and increased numbers of lawsuits is not small thing:
The FDA panel could simply approve the plan if it should find it carried great scientific weight, said Dr. Douglas Diekema, director of medical ethics at Children's Hospital in Seattle.
New Jersey attorney Alan C. Milstein said that would be the wrong call.
Milstein, who represented the family of an 18-year-old whose death in 1999 spurred greater federal oversight of gene therapy trials, pointed to a recent Maryland Supreme Court ruling. The court held that exposing healthy children to higher-than-minimum risk in a medical study is unethical.
"They can't do this study. It doesn't take a genius to figure out why they can't do it," Milstein said. "I can't believe that anybody is going to say it's ethical to do this. It's not even a close call."
Extensive briefing materials for the Pediatric Ethics Subcommittee's Sept. 10 meeting are
here. And
a recent review article in the August 28 issue of
The Lancet (Caldwell
et al., "Clinical Trials in Children," Lancet 2004;364:803-11 [subscription required]) takes a good look at the topic from all sides. Here's the abstract:
The imperative to undertake randomised trials in children arises from extraordinary advances in basic biomedical sciences, needing a matching commitment to translational research if child health is to reap the benefits from this new knowledge. Unfortunately, many prescribed treatments for children have not been adequately tested in children, sometimes resulting in harmful treatments being given and beneficial treatments being withheld. Government, industry, funding agencies, and clinicians are responsible for research priorities being adult-focused because of the greater burden of disease in adults, coupled with financial and marketing considerations. This bias has meant that the equal rights of children to participate in trials has not always been recognised. This is changing, however, as the need for clinical trials in children has been increasingly recognised by the scientific community and broader public, leading to new legislation in some countries making trials of interventions mandatory in children as well as adults before drug approval is given. Trials in children are more challenging than those in adults. The pool of eligible children entering trials is often small because many conditions are uncommon in children, and the threshold for gaining consent is often higher and more complex because parents have to make decisions about trial participation on behalf of their child. Uncertain about what is best, despite supporting the notion of trials in principle, parents and paediatricians generally opt for the new intervention or for standard care rather than trial participation. In this review, we explore issues relating to trial participation for children and suggest some strategiesfor improving the conduct of clinical trials involving children.
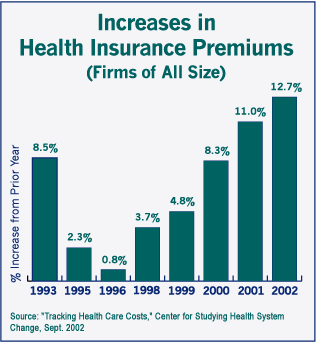 An article in today's Wall Street Journal (click here - link should be good for about a week) reports that "Americans should expect to pay more for medical costs whether they are employed or retired, according to two new studies. The reports, by Milliman Inc. and Watson Wyatt Worldwide, show that health-care costs are still rising at a fast pace -- despite slowing from double-digit rates in recent years -- and that businesses expect to curtail or limit retiree medical benefits."
An article in today's Wall Street Journal (click here - link should be good for about a week) reports that "Americans should expect to pay more for medical costs whether they are employed or retired, according to two new studies. The reports, by Milliman Inc. and Watson Wyatt Worldwide, show that health-care costs are still rising at a fast pace -- despite slowing from double-digit rates in recent years -- and that businesses expect to curtail or limit retiree medical benefits."