
Tuesday, August 29, 2006
"Medically inappropriate treatment" - how do we decide?
 There's a good piece in the NY Times today about dialysis, when and how it should be withheld, and who should decide: "Choosing a 'God Squad,' When the Mind Has Faded" by Barron Lerner, MD. Here's how it starts:
There's a good piece in the NY Times today about dialysis, when and how it should be withheld, and who should decide: "Choosing a 'God Squad,' When the Mind Has Faded" by Barron Lerner, MD. Here's how it starts:Would you want your tax dollars to pay for dialysis for a patient with irreversible brain damage? In 1972, when Congress agreed to use Medicare money to finance dialysis for patients with end-stage kidney failure, this question had never come up.
But now, new research shows, many patients on dialysis have severe mental impairment. Is it appropriate, or even possible, to refuse to give patients this treatment?
The article mentions Medicare's End-Stage Renal Dialysis program, which covers dialysis for all who are medically qualified to receive it; the article then takes a trip down the resource-allocation highway, followed by an abrupt turn down a more patient-centered boulevard:
But there were new problems. For one, the bill’s sponsors underestimated the demand for dialysis, now given to more than 300,000 patients a year, at a cost of more than $16 billion. It also became clear that the technology was, in some cases, being used indiscriminately.
In 2000, Dr. Alvin H. Moss, director of the Center for Health Ethics and Law at West Virginia University, led a committee of the Renal Physicians Association and the American Society of Nephrology that developed guidelines on the use of dialysis. It was found to be inappropriate for those with “irreversible, profound neurological impairment,” among others. The committee also said it was reasonable to consider withholding dialysis from patients with terminal illnesses unrelated to the kidneys. . . .
“The renal-care team has the right to refuse to offer dialysis when the expected benefits do not justify the risks,” Dr. Moss said. At his home institution, Dr. Moss is taking a more hard-line approach, saying no to families who request what he believes is inappropriate dialysis. At other times, he offers the dialysis, but if the patient doesn’t improve, it is stopped.
So far he has not been sued, he said, citing thoughtful discussions he has had with family members about what dialysis can and cannot achieve.
But the fear of lawsuits continues to worry many nephrologists who believe that it is safer to provide dialysis. And there remains that old American unwillingness to let people die, even when it is surely their time.
Dr. Valeri, of Columbia, knows this feeling well. If he suggests to relatives that dialysis be withheld for a gravely ill family member, they confuse it with euthanasia. “They think you are just another Kevorkian,” he said.
In a microcosm, this is exactly the discussion we've been having in Texas this year, as we debate the merits of Texas' so-called "futility law," which allows hospitals to stop life-sustaining treatment when a physician says the treatment is not appropriate and an ethics committee agrees (§ 166.046 of the Health & Safety Code). Are there limits beyond which otherwise appropriate care becomes inappropriate? What is the source of those limits: benefits/burdens ratio for the patient? benefits/burdens for society? a professional ethos among nephrologists (pulmonologists, cardiologists, intensivists, et al.)? Is the threat of litigation a good thing or a bad thing? What do we mean by good end-of-life care? If the technological imperative is resisted, is that euthanasia or the wise practice of medicine?
Friday, August 25, 2006
New from the law reviews
 Courtesy of the Marian Gallagher Law Library at the University of Washington:
Courtesy of the Marian Gallagher Law Library at the University of Washington:A. FOOD AND DRUG LAW
- Cooper, Tracy. Picture this: promoting sustainable fisheries through eco-labeling and product certification. 10 Ocean & Coastal L.J. 1-49 (2004-2005). [L][W]
- Levy, Elissa. Note. The HEALTH Act's FDA defense to punitive damages: a gift to drug makers or to the public? 74 Fordham L. Rev. 2425-2460 (2006). [L][W]
- Globalization of Pharmaceuticals: International Regulatory Issue. Foreword by Frances H. Miller; articles by Kevin Outterson, John A. Vernon, Joseph H. Golec, W. Keener Hughen, Aidan Hollis, Peter Ibbott, Mary Ellen Fleck Kleiman, Daniel Gilman, Bryan A. Liang, Donald deKieffer, Robert Gatter and W. John Thomas. 32 Am. J.L. & Med. 153-380 (2006) [L][W]
B. HEALTH LAW AND POLICY
- Gitter, Donna M. Am I my brother's keeper? The use of preimplantation genetic diagnosis to create a donor of transplantable stem cells for an older sibling suffering from a genetic disorder. 13 Geo. Mason L. Rev. 975-1035 (2006). [L][W]
- Levy, Elissa. Note. The HEALTH Act's FDA defense to punitive damages: a gift to drug makers or to the public? 74 Fordham L. Rev. 2425-2460 (2006). [L][W]
- McMullen, Judith G. Underage drinking: does current policy make sense? 10 Lewis & Clark L. Rev. 333-365 (2006). [L][W]
- Schumaker, Anna C. Rules were not meant to be broken: alleviating the tension between privacy and discovery in medical record disputes. 40 Val. U. L. Rev. 845-896 (2006). [L][W]
- Wyatt, Mike J. Comment. Buy out or get out: why covenants not to compete in surgeon employment contracts are truly bad medicine. (Idbeis v. Wichita Surgical Specialists, P.A., 112 P.3d 81, Kan. 2005.) 45 Washburn L.J. 715-739 (2006). [L][W]
- Effron, Robin Jane. Dependence, identity, and abortion politics. 1 NYU J.L. & Liberty 1108-1133 (2005). [L][W]
- Grissom, Charlotte D. Note. A balance between life and death: a look at the government's role in the right to die debate. 49 How. L.J. 615-642 (2006). [L][W]
- Martin, Laura. Comment. Civil procedure: procedural due process does not toll the Tennessee medical malpractice statute of repose. (Mills v. Wong, 155 S.W.3d 916, Tenn. 2005.) 36 U. Mem. L. Rev. 805-827 (2006). [L][W]
- Siegel, Reva B. You've come a long way, baby: Rehnquist's new approach to pregnancy discrimination in Hibbs. 58 Stan. L. Rev. 1871-1898 (2006). [L][W]
[L] = Lexis/Nexis link
[W] = WestLaw link
AHLA's Health Lawyers Weekly (Aug. 25)
 Here's the table of contents from today's Health Lawyers Weekly, reprinted here with the kind permission of the AHLA:
Here's the table of contents from today's Health Lawyers Weekly, reprinted here with the kind permission of the AHLA:Top Stories
- Bush Signs Executive Order Requiring Federal Agencies To Increase Price And Quality Transparency
President George W. Bush signed August 22 an executive order directing federal agencies that administer or sponsor a healthcare program to increase price and quality transparency by January 1, 2007. Full Story - OIG Publishes Guidelines For Evaluating State FCA Cases
The Department of Health and Human Services Office of Inspector General (OIG) issued a notice in the August 21 Federal Register (71 Fed. Reg. 48552) announcing the publication of OIG’s guidelines for evaluating state False Claims Act cases. Full Story
Articles & Analyses
- Implementing A Trusted Health Information Exchange, By Zoë Baird, President Marke Foundation
- 2005-2006 Teaching Hospitals And Academic Medical Centers Year In Review, Compiled by Health Lawyers' Teaching Hospitals and Academic Medical Centers Practice Group
Current Topics
- Antitrust
FTC Says IPAs Engaged In Anticompetitive Conduct - Criminal Law
Fourth Circuit Says Plaintiff Bound To Appeal Waiver In Plea Agreement - Employment and Labor
1. Eighth Circuit Finds Terminated Physician Did Not Qualify As Whistleblower
2. U.S. Court In Tennessee Says Termination Of Tenured Faculty Members Violated Due Process - EMTALA
U.S. Court In Alabama Allows Pregnant Woman’s EMTALA Claim To Go Forward - ERISA
Fifth Circuit Holds ERISA Does Not Preempt Louisiana Assignment Statute - Food and Drug Law
1. U.S. Court In California Says Federal Law Preempts Pharmaceutical Failure-To-Warn Claims
2. FDA Proposes Comprehensive Electronic Drug Registration List
3. FDA Approves Plan B For OTC Use - Fraud and Abuse
1. DOJ Announces $20 Million Settlement Of FCA Charges
2. Seventh Circuit Finds Qui Tam Relator Must Show Specific Claim That Was False
3. North Carolina To Repay Federal Government $151.5 Million Of Medicaid Reimbursements
4. Update - Hospitals and Health Systems
USC Seeks To Part Ways With Tenet Subsidiary - Medicaid
NASMD, APHSA Urges DHHS Not To Implement Proposed Medicaid Regulatory Changes - Medicare
1. CMS Posts Medicare Payment Data On Common ASC Procedures
2. Eighth Circuit Says Hospital’s Classroom Costs Not Entitled To Pass-Through Treatment
3. Study Finds Variation In Part D Plans’ Drug Coverage - News in Brief
CMS Solicits Proposals For New Risk Reduction Demonstration
(c) 2006, AHLA. Reprinted with permission.
OTC sales of Plan B approved for adults
 After years of hassling over whether to approve over-the-counter sales of the Plan B contraceptives, the FDA has finally relented and announced yesterday that the "morning after" pill would become available for purchase by adults by the end of the year. (NY Times; Wall Street Journal; Washington Post; AP/MyWay) Compared to the original application three years ago, which sought approval for sales by under-18's as well, this is something of a compromise, and a disappointment to advocates for broader access. Much of the agency's foot-dragging was wrapped in official comments that questioned the adequacy of safety data for teenaged users, although critics of the agency believed the doubts were a smokescreen for the Bush administration, which appeared to opposed broadening the availability of this contraceptive on political grounds. Their bottom line -- on a number of issues -- seems to be that they are against anything that might make teen sex a little less dangerous and therefore a little more attractive: "Plan A (Abstinence) or the highway!" Could a government policy be more backward? At least yesterday's announcement is a start. Meanwhile, according to the AP, "[t]he Center for Reproductive Rights said a lawsuit filed last year to do away with all age restrictions would continue."
After years of hassling over whether to approve over-the-counter sales of the Plan B contraceptives, the FDA has finally relented and announced yesterday that the "morning after" pill would become available for purchase by adults by the end of the year. (NY Times; Wall Street Journal; Washington Post; AP/MyWay) Compared to the original application three years ago, which sought approval for sales by under-18's as well, this is something of a compromise, and a disappointment to advocates for broader access. Much of the agency's foot-dragging was wrapped in official comments that questioned the adequacy of safety data for teenaged users, although critics of the agency believed the doubts were a smokescreen for the Bush administration, which appeared to opposed broadening the availability of this contraceptive on political grounds. Their bottom line -- on a number of issues -- seems to be that they are against anything that might make teen sex a little less dangerous and therefore a little more attractive: "Plan A (Abstinence) or the highway!" Could a government policy be more backward? At least yesterday's announcement is a start. Meanwhile, according to the AP, "[t]he Center for Reproductive Rights said a lawsuit filed last year to do away with all age restrictions would continue."
Thursday, August 24, 2006
Why is 16% of GDP too much to spend on health care?
 That will be one of my questions tomorrow in the first class in Health Law. I remember, back during the debate over the Clintons' plan, the tongue-in-cheek report that, at the then-current rate of health-care inflation, in 50 years 100% of our GDP would be health care ("every man, woman, and child would be in hospital beds administering IVs to one another"). Now, quite sensible people (e.g., Robert W. Fogel, a Nobel laureate at the University of Chicago's Graduate School of Business) predict that health care will account for 25% of GDP by 2030. It's less than 100%, but it's still the kind of number that makes you sit up and take notice.
That will be one of my questions tomorrow in the first class in Health Law. I remember, back during the debate over the Clintons' plan, the tongue-in-cheek report that, at the then-current rate of health-care inflation, in 50 years 100% of our GDP would be health care ("every man, woman, and child would be in hospital beds administering IVs to one another"). Now, quite sensible people (e.g., Robert W. Fogel, a Nobel laureate at the University of Chicago's Graduate School of Business) predict that health care will account for 25% of GDP by 2030. It's less than 100%, but it's still the kind of number that makes you sit up and take notice.It also made Gina Kolata sit up and take notice in Tuesday's New York Times. There is much to think about (and discuss in class) in this article, including this exchange:
Unless the current system is changed, most health care costs will continue to be paid by insurance, especially Medicare, which means that the taxpayers will foot the bill. But Dr. Fogel says he is not alarmed. Americans can afford it, he says, because the nation is so rich.
“It takes so little of household income to satisfy expenditures on food, clothing and shelter,” he explains. “At the end of the 19th century, food, clothing and shelter accounted for 80 percent of the family budget. Today it’s about a third.” Other economists agree. “We have to spend our money on something,” says Robert E. Hall, a Stanford University economist.
Wednesday, August 23, 2006
New technique for deriving embryonic stem cells that doesn't destroy the embryo
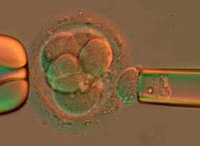 You would think that a technique that allows lab techs to grow embryonic stem cell lines without destroying the embryo would be the ultimate answer to the principal objection to embryonic stem cell research. But you would be wrong.
You would think that a technique that allows lab techs to grow embryonic stem cell lines without destroying the embryo would be the ultimate answer to the principal objection to embryonic stem cell research. But you would be wrong.An on-line letter (1st paragraph only) at the journal Nature (requires subscription) describes the technique, as do articles posted this afternoon to the web sites of the New York Times and the Wall Street Journal (requires subscription). Researchers at Advanced Cell Technology report success borrowing the technique used for pre-implantation genetic diagnosis ("PGD") of embryos created at in vitro fertilization centers. The technique takes the fertilized egg at the point that it is an 8-celled organism. The cells are called blastomeres, and PGD removes one blastomere for genetic testing and screening. Now 10 years old, PGD produces no discernible harm to the remaining 7-cell organism, which appears capable of developing into a normal, health embryo and then fetus. It was reported last year that embryonic stem cells were derived from mouse embryos using this technique. The ACT letter appears to be the first report that the technique can be successfully performed on human embryos. ACT's press release is here. More details are also available from the statement issued by ACT's ethics advisory board.
Despite the head-on challenge this technique represents to current ethical objections to harvesting embryonic stem cells, both papers report that the news was met with different degrees of skepticism, dismay, and downright hostility by the U.S. Conference of Catholic Bishops ("gravely unethical" -- the bishops also oppose IVF and PGD), Glenn McGee ("this will please no one" -- McGee is described as a long-time critic of ACT), and the immediate past chair of the President's Council on Bioethics, Leon Kass ("'I do not think that this is the sought-for, morally unproblematic and practically useful approach we need.' He said the long-term risk of P.G.D. testing is unknown, and that the present stem-cell technique is inefficient, requiring blastomeres from many embryos to generate each new cell line. It would be better to derive human stem cell lines from the body’s mature cells, he said, a method that researchers are still working on.")
Sunday, August 20, 2006
Internet prescribing legislation introduced in U.S. Senate
 From the Federation of State Medical Boards:
From the Federation of State Medical Boards:New legislation designed to regulate the sale of prescription drugs and controlled substances over the Internet was introduced in the U.S. Senate on Aug. 10. The “Online Pharmacy Consumer Protection Act of 2006” (SB 3834)would:
- Prohibit the distribution of controlled substances and prescription drugs via the Internet without a valid prescription issued for a legitimate medical purpose in the usual course of professional practice that is based upon a qualifying medical relationship by a practitioner
- Provide criminal penalties for unlawfully dispensing controlled substances and prescription drugs over the Internet
- Give state attorneys general a civil cause of action against violators
- Allow the federal government to take possession of property used illegally by online pharmacies
- Require online pharmacies to file an additional registration statement with the attorney general and meet additional registration requirements
- Report to the attorney general all controlled substances and prescription drugs dispensed over the Internet
Saturday, August 19, 2006
Latest from AHLA's Health Lawyers Weekly (18 Aug 2006)
 Herewith, the table of contents of this week's American Health Lawyers' Health Lawyers Weekly (a free member benefit of AHLA):
Herewith, the table of contents of this week's American Health Lawyers' Health Lawyers Weekly (a free member benefit of AHLA):(c) 2006. Reprinted by permission of AHLA.Top Stories
- CMS Issues Final Quality Standards For DMEPOS Suppliers
The Centers for Medicare and Medicaid Services (CMS) released August 14 its final quality standards for suppliers of durable medical equipment, prosthetics, orthotics, supplies, (DMEPOS) and other items and services under the Medicare program. The standards have been scaled-back substantially from the draft version issued in September 2005, thereby reducing the standards document from 104 pages to fourteen pages. Full Story- OIG Finds Some MA Marketing Materials Not In Compliance With CMS Requirements
Some Medicare Advantage (MA) marketing materials for 2005 did not meet the Centers for Medicare and Medicaid Services' (CMS') requirements for marketing, the Department of Health and Human Services Office of Inspector General (OIG) found in a report issued August 14. Full StoryArticles & Analyses
- Impact Of Proposed Changes To The Medicare Physician Fee Schedule On Diagnostic Imaging Providers
By Thomas W. Greeson and Heather M. Zimmerman, Reed Smith LLP, Falls Church, VA- 2005-2006 Tax And Finance Year In Review
Compiled by Health Lawyers' Tax and Finance Practice GroupCurrent Topics
- AIDS
New Jersey Appeals Court Finds Hospital That Failed To Notify Patient Of Positive HIV Test Results Civilly Liable To Patient's Sexual- Employment and Labor
1. North Carolina Appeals Court Finds Covenant Not To Compete Valid
2. Missouri Supreme Court Finds Home Healthcare Provider's Noncompetition Agreements Are Valid And Enforceable- ERISA
U.S. Court In Ohio Finds ERISA Does Not Preempt Hospital's State Law Claims Against Plan Administrator- Food and Drug Law
1. OIG Report Finds Deficiencies In FDA's National Drug Code Directory
2. FDA Issues Warning Letters To Three Pharmacies Engaged In Compounding Mass Amounts Of Inhalation Drugs- Fraud and Abuse
1. Louisiana Hospital Agrees To Pay U.S. $3.8 Million To Settle Fraud Allegations
2. Update- Health Policy
Many Americans Report Unsafe Or Wasteful Healthcare, New Survey Finds- Healthcare Access
Study Finds Fewer Physicians Accepting New Medicaid Patients- Medicaid
1. Nebraska Supreme Court Finds Longer Look-Back Period Applied To Medicaid Eligibility Decision Was Error
2. House Members Urge CMS To Change Medicaid Citizenship Requirements- Medical Malpractice
1. Connecticut Appeals Court Finds Patient's Medical Malpractice Action Against Radiologist Time-Barred
2. Louisiana Appeals Court Finds Lack Of Notation In Medical Record Created Material Issue Regarding Whether Treatment Recommendation Was Made To Patient- Medical Records
Mississippi Supreme Court Finds Health Insurer Did Not Owe Fiduciary Duty To Maintain Confidentiality Of Insured's Medical Records- Medicare
Part D Costs For 2007 Lower Than Predicted, CMS Says- Physicians
Iowa Appeals Court Finds Evidence Supports Medical Board's Disciplinary Action- Quality of Care
NCQA Seeking Input On Implementation Of Single Set Of Standards For All Health Plans
Friday, August 18, 2006
Money, money, money . . . and the occasional whistleblower
 The post below about suspiciously high rates of angioplasties at hospitals in Elyria, Ohio, reminds me of three other items in the news recently (also from Modern Healthcare):
The post below about suspiciously high rates of angioplasties at hospitals in Elyria, Ohio, reminds me of three other items in the news recently (also from Modern Healthcare):- Cleveland Clinic severs ties with cardiologist-inventor
The Cleveland Clinic [link] said it "took action not to reappoint" cardiologist Jay Yadav [link - but probably not for long] to its staff, reportedly because Yadav failed to disclose financial interests in a stroke-prevention product he helped invent and market. "There were some disclosure issues that led our board of governors to act," said Eileen Sheil, the clinic's executive director of public and media relations. The clinic initiated an outside review of its conflict-of-interest policies in December 2005 following a Wall Street Journal report that raised questions about relationships between the clinic's financial interests and research conducted by its physicians on patients. In May, the clinic adopted new policies that included a "renewed commitment by the board to maintain and preserve a balance between innovation and transparency," according to a statement. Yadav, 46, joined the clinic in 1998 and was named chairman of Cleveland Clinic Foundation Innovations, its technology transfer and commercialization division, in October 2005. AngioGuard, a company Yadav founded prior to joining the clinic, was sold to Cordis Corp., a division of Johnson & Johnson, in 1999 for about $40 million, plus royalties, according to the Cleveland Plain Dealer. Yesterday, Yadav offered to donate his proceeds from the deal to charity, the Plain Dealer reported. At deadline, Yadav and a spokesperson for Cordis could not be reached for comment. - N.C., hospitals to repay $151 million to Medicaid
North Carolina and 51 hospitals in the state will repay the federal government a total of $151.5 million in alleged overpayments from the Medicaid disproportionate-share program. "While substantial overpayments have been made to the state, and, in turn, to a large number of hospitals within the state, no evidence of criminal wrongdoing or civil fraud has been uncovered," federal authorities said. The overpayments took place between 1997 and 2003, and an initial analysis questioned some $400 million in disproportionate-share distributions and the role several hospitals played in the matter. In a news release today, investigating agencies said the state used "an overly aggressive plan to obtain the maximum amount of federal healthcare dollars," made significant accounting errors and failed to ensure "timely cost settlements." The 51 hospitals will contribute $91 million of the total repayment, which will be made over four years. A spokesman for the North Carolina Hospital Association said hospitals are "pleased to be totally vindicated ... The state simply didn't have the resources to run this program." Remember, improper DSH payments were part of the sordid story at University Hospital in New Jersey. - La. hospital settles case alleging unnecessary care
Our Lady of Lourdes Regional Medical Center, Lafayette, La., will pay $3.8 million to settle civil False Claims Act allegations that the 264-bed hospital allowed a cardiologist to bill for unnecessary angiograms, angioplasties and other procedures and financially benefited from doing so. The cardiologist, Mehmood Patel, is scheduled to go to trial in March 2007 on criminal Medicare fraud charges. He has pleaded not guilty. The whistle-blower, physician Christopher Mallavarapu, will receive $760,000 of the civil settlement. According to the complaint, Mallavarapu said he reported his concerns to hospital officials and was told they would take no action. Lourdes' president and chief executive officer, W.F. "Bud" Barrow, said in a statement that the hospital suspended Patel's privileges after investigating questionable practices and reported its concerns to HHS' inspector general's office and the U.S. attorney's office. Lourdes signed a five-year corporate integrity agreement as part of the settlement but didn't admit wrongdoing. At deadline, Patel could not be reached for comment.
Connect the dots . . . .
It's a good time to be in cardiology
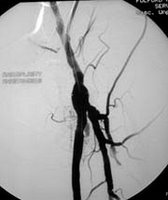 Two items from the print press, courtesy of Modern Healthcare's "Daily Dose":
Two items from the print press, courtesy of Modern Healthcare's "Daily Dose":- In Philadelphia, heart-transplant centers abound (Philadelphia Inquirer)
After a massive heart attack last year, doctors told David Kaminstein that he needed a transplant. He had the choice of five hospitals in the Philadelphia area that could do the complicated operation. That's a lot of choices -- some say too many. With the launch of two new heart-transplant centers in recent years, this region has the same number of programs as Los Angeles, though just half the population. Last year, only the Hospital of the University of Pennsylvania -- with 49 transplants -- performed more than 13 of the operations. Most healthcare experts say that the more patients a transplant team treats, the better the results. - Angioplasty rates off the charts in Ohio city (New York Times)
People with blocked coronary arteries can typically choose among drugs, bypass surgery and vessel-clearing procedures like angioplasty. But in Elyria, a small, aging industrial city in northeast Ohio, doctors are much more likely than those anywhere else in the country to steer patients toward angioplasty. No one has accused the doctors involved of any wrongdoing. But the statistics are so far off the charts -- Medicare patients in Elyria receive angioplasties at a rate nearly four times the national average -- that Medicare and at least one commercial insurer are starting to ask questions. And the hospital where most of the procedures take place says it plans to conduct an independent review.
Fresh from the law reviews
 From the Gallagher Law Library at the University of Washington:
From the Gallagher Law Library at the University of Washington:- Carroll, Allison N. Note. Mandatory nurse-to-patient ratios: can ratios bring better healthcare to Kentucky? 44 Brandeis L.J. 673-693 (2006). [L][W]
- Jordan, Christina. Note. The XXX-files: Cal/OSHA's regulatory response to HIV in the adult film industry. 12 Cardozo J.L. & Gender 421-444 (2005). [L][W]
- Ono, Nahoko. Better than nothing: Japan's next move on patentability of medical methods. 37 IIC 196-218 (2006). [L][W]
- Symposium: Precious Commodities: The Supply & Demand of Body Parts. Introduction by Michele Goodwin; articles by Lloyd R. Cohen, T. Randolph Beard, David L. Kaserman, Steven H. Resnicoff, William E. Stempsey, David J. Undis, Raymond Pollak, Michelle Oberman, Robert A. Katz, Kieran Healy and Elisa J. Gordon. 55 DePaul L. Rev. 793-1066 (2006). [L][W]
- Deutsch, Josh. Note. Finder-keepers: a bright-line rule awarding custody to gestational mothers in cases of fertility clinic error. 12 Cardozo J.L. & Gender 367-389 (2005). [L][W]
- Kotur, Kelly. Student work. An extreme response or a necessary reform? Revealing how caps on noneconomic damages actually affect medical malpractice victims and malpractice insurance rates. 108 W. Va. L. Rev. 873-900 (2006). [L][W]
- Mahoney, Kathleen A. Note. Malpractice claims resulting from negligent preconception genetic testing: do these claims present a strain of wrongful birth or wrongful conception, and does the categorization even matter? 39 Suffolk U.L. Rev. 773-793 (2006). [L][W]
- Van Tassel, Katharine. Hospital peer review standards and due process: moving from tort doctrine toward contract principles based on clinical practice guidelines. 36 Seton Hall L. Rev. 1179-1256 (2006). [L][W]
- Note. After Ayotte: the need to defend abortion rights with renewed "purpose". 119 Harv. L. Rev. 2552-2573 (2006). [L][W]
- Recent development. Federal and Florida judicial and legislative involvement in the Theresa Schiavo case. Health care law--treatment--privacy rights--due process--withdrawal of life support--the Theresa Schiavo decisions. 33 Fla. St. U. L. Rev. 356-371 (2005). [L][W]
"L" = Lexis/Nexis link; "W" = WestLaw link
Wednesday, August 16, 2006
Medical liability insurers profiting handsomely in wake of Texas tort reform
 Three years after tort reform hit the books in Texas, the state's medical liability insurers have lowered premiums somewhat and added enormously to their bottom line, according to a story in the August 11 issue of the Austin Business Journal. The largest of them all -- Texas Medical Liability Trust -- has shown the greatest gains:
Three years after tort reform hit the books in Texas, the state's medical liability insurers have lowered premiums somewhat and added enormously to their bottom line, according to a story in the August 11 issue of the Austin Business Journal. The largest of them all -- Texas Medical Liability Trust -- has shown the greatest gains:The state's largest medical malpractice insurer -- Texas Medical Liability Trust, which is based in Austin -- may have the best post-tort reform success story.
In its 2005 annual report, TMLT detailed how, in just five years since 2001, its surplus has gone from $22.9 million to $203.4 million -- an increase of almost 800 percent. Over the same period, its assets almost doubled, going from $333.9 million in 2001 to $588.7 million last year. During the same time, however, its insurance losses went down by almost half, from $137.2 million in 2001 to $73.2 million last year.
And, the article continues, "Texas' second-largest doctor insurer, Fort Wayne, Ind.-based Medical Protective Corp., is doing well enough that last year it was bought by Berkshire Hathaway Inc., the legendary company run by the world's second-richest man, Warren Buffett."
Tuesday, August 15, 2006
More on emergency room practices
 This from the East Bay Business Times in California . . . . Sutter Delta Medical Center, among others in the region, has cut waiting times in its ER from 4-6 hours to 1-2 hours. They've done it by being imaginative in their triaging of patients, getting noncritical patients to doctors faster than before, for instance. The hospital's director of emergency services says, "People are still using the ER as their primary-care provider. Our challenge was what kind of mechanism could we use to treat the nonemergency patients." Beats the daylights out of a brochure and a boot to the butt.
This from the East Bay Business Times in California . . . . Sutter Delta Medical Center, among others in the region, has cut waiting times in its ER from 4-6 hours to 1-2 hours. They've done it by being imaginative in their triaging of patients, getting noncritical patients to doctors faster than before, for instance. The hospital's director of emergency services says, "People are still using the ER as their primary-care provider. Our challenge was what kind of mechanism could we use to treat the nonemergency patients." Beats the daylights out of a brochure and a boot to the butt.
ER sends nonemergency patients packing
 This might be a case of "dog bites man," but the Jacksonville Business Journal reports that area HCA hospitals have adopted the practice of screening emergency room patients (as required by EMTALA) and showing nonemergency patients the door (as permitted by EMTALA) with a brochure listing area clinics in their hands. Is this news, exactly? In my limited urban ER experience, you can sit in the waiting room on a Saturday or Sunday morning and watch one person after another get screened by a nurse who shows the BP/temp/pulse data to a doc and then gently but firmly escorts the almost-a-patient back out to the street. The more humane or enlightened institutions have an ambulatory care clinic to which the a-a-p can be directed, though most ACC's seem to be open only during regular business hours Mon.-Fri. Another common practice is to triage nonemergency ER patients to a non-acute treatment room within the emergency department. The brochure is a nice touch, though the article does quote someone who says the brochure has several inaccurate phone numbers and facility names.
This might be a case of "dog bites man," but the Jacksonville Business Journal reports that area HCA hospitals have adopted the practice of screening emergency room patients (as required by EMTALA) and showing nonemergency patients the door (as permitted by EMTALA) with a brochure listing area clinics in their hands. Is this news, exactly? In my limited urban ER experience, you can sit in the waiting room on a Saturday or Sunday morning and watch one person after another get screened by a nurse who shows the BP/temp/pulse data to a doc and then gently but firmly escorts the almost-a-patient back out to the street. The more humane or enlightened institutions have an ambulatory care clinic to which the a-a-p can be directed, though most ACC's seem to be open only during regular business hours Mon.-Fri. Another common practice is to triage nonemergency ER patients to a non-acute treatment room within the emergency department. The brochure is a nice touch, though the article does quote someone who says the brochure has several inaccurate phone numbers and facility names.
Monday, August 14, 2006
Kaiser fined for mismanagement of its kidney-transplant program
 Considering how neurotic the organ-transplant industry is to maintain a squeaky-clean image, it's remarkable that Kaiser Permanente's been hit with a massive fine from California's Department of Managed Health Care [press release] for mismanaging its kidney-transplant program. Even more significant, for my money, is the lesson here.
Considering how neurotic the organ-transplant industry is to maintain a squeaky-clean image, it's remarkable that Kaiser Permanente's been hit with a massive fine from California's Department of Managed Health Care [press release] for mismanaging its kidney-transplant program. Even more significant, for my money, is the lesson here.How many times has a health care provider tried to minimize the imposition of civil penalties by characterizing its lapses as "mere" record-keeping or bureaucratic errors, insisting all the while that no patient was put at risk and quality care wasn't compromised? My take on such evasions is that paperwork snafu's are typically the tip of the iceberg or (to mix my metaphors) the regulator's low-hanging fruit. If a place can't keep the administrative details straight, you can bet there's more to the situation than misplaced files and incomplete reports. Kaiser's situation is a good example:
Kaiser suspended its Northern California kidney transplant program in May amid mounting regulatory pressure and patient lawsuits alleging that botched paperwork and administrative errors had imperiled lives.
Problems arose when Kaiser ordered Northern California kidney patients to transfer from University of California hospitals to its new transplant center in 2004.
Kaiser failed to discuss with regulators the transfer of up to 1,500 patients to the new center, delaying some patients' procedures, the Los Angeles Times reported. Only 56 transplants were performed at the Kaiser's San Francisco center in 2005, while twice that number of people died waiting for a kidney, the Times reported.
At other California transplant centers, more than twice as many people received kidneys than died.
Lucinda Ehnes, director of the managed care department, said Kaiser's administrative oversight was inadequate and it provided too few personnel to accomplish the transfers.
The company also failed to provide timely access to specialists and did not properly respond to patient complaints, she said.
Mary Ann Thode, president of Kaiser Foundation Health Plan and Hospitals in the Northern California region, said the HMO deeply regretted "any problems, difficulties or concerns we may have caused any of our patients as a result of their experience."
"Problems," "difficulties," and "concerns" are corporate euphemisms for the likely loss of lives of patients who placed their faith in Kaiser. But give Kaiser credit: it isn't engaging in the usual evasions about "mere errors in the paperwork," but is instead taking responsibility and vowing to do better in the future.
Costly Drugs Force Life-Death Decisions
 From the AP, a good story about the costly, high-tech armamentorium of drugs and devices that offer the promise of extending life-spans once deemed to be "terminal," but at a price that's so high, some patients simply opt out:
From the AP, a good story about the costly, high-tech armamentorium of drugs and devices that offer the promise of extending life-spans once deemed to be "terminal," but at a price that's so high, some patients simply opt out:More patients are confronting this wrenching decision, as the latest generation of pricier cancer drugs and heart implants stretches out the final months of advanced disease. Is the chance for several more months of life - maybe a year or more with luck - precious enough to spend a small fortune? This dilemma is also challenging governments, employers and insurers, who all help finance America's longer life spans and innovative technologies.
Extraordinary care for dying patients can make for inspiring medicine, but its extraordinary costs make it an increasingly debated choice to promote public health. Many economists, doctors, and ethicists say this care too often buys too little for too much - and that its expanding share of medical resources might better pay for screening and treating diseases in earlier stages.
Already, up to 30 percent of annual payments by federal Medicare insurance go to the 5 percent of members in their last year of life, research shows.
"People still have an underlying belief that there's an infinite amount of resources that can be invested in health care," says Dr. Harlan Krumholz, a Yale University heart specialist who studies quality of care. "But I think we're coming to a realization that we're going to need to confront these issues explicitly."
Maybe so, but any retreat from last-resort care still raises objections from many patients, doctors and medical companies. They denounce "rationing" of care and defend expensive treatments for the dying as a moral imperative.
Sunday, August 13, 2006
More on non-heart-beating organ donors
 "NHBD" is slowly being replaced by "DCD" ("donation after cardiac death"), but whatever name it goes by, these organ-donor protocols continue to get (deservedly) close scrutiny, most recently in the New Scientist. The move away from brain death and toward cardiopulmonary death is, contrary to the implications of this article, not evidence of a "new" standard for determining when death occurs, but rather is a return to the old way of determining death. Most states retained cardiopulmonary death when they adopted brain death, so no change in law is needed except in the few states that made neurological criteria the sole standard for determining when death occurs. The real concerns -- and this is brought out in the article -- are over whether the existing protocols run roughshod over the the requirement that the absence of cardiac and pulmonary function be irreversible and whether the administration of regitine and heparin cause the cessation of cardiopulmonary function. For more on all this, a good place to start is the 1997 IOM report, "Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement."
"NHBD" is slowly being replaced by "DCD" ("donation after cardiac death"), but whatever name it goes by, these organ-donor protocols continue to get (deservedly) close scrutiny, most recently in the New Scientist. The move away from brain death and toward cardiopulmonary death is, contrary to the implications of this article, not evidence of a "new" standard for determining when death occurs, but rather is a return to the old way of determining death. Most states retained cardiopulmonary death when they adopted brain death, so no change in law is needed except in the few states that made neurological criteria the sole standard for determining when death occurs. The real concerns -- and this is brought out in the article -- are over whether the existing protocols run roughshod over the the requirement that the absence of cardiac and pulmonary function be irreversible and whether the administration of regitine and heparin cause the cessation of cardiopulmonary function. For more on all this, a good place to start is the 1997 IOM report, "Non-Heart-Beating Organ Transplantation: Medical and Ethical Issues in Procurement."
Latest from AHLA's Health Lawyers Weekly (11 Aug 2006)
 With the permission of the American Health Lawyers Association, here's this week's Table of Contents for its Health Lawyers Weekly (free member benefit):
With the permission of the American Health Lawyers Association, here's this week's Table of Contents for its Health Lawyers Weekly (free member benefit):Top Stories
- CMS Projects 5.1% Reduction In Medicare Physician Payment Rates For 2007: The Centers for Medicare and Medicaid Services (CMS) is projecting a negative 5.1% update in the Medicare physician fee schedule for 2007 under the Sustainable Growth Rate (SGR) formula, according to a proposed rule released August 8.Under the proposal, CMS expects payments of roughly $61.5 billion to 875,000 physicians and other healthcare professionals in 2007. Full Story
- CMS Issues Proposed OPPS Rule Containing Significant Revisions To ASC Payment: Hospitals will receive an overall average increase in Medicare payments of 3% in calendar year (CY) 2007 for outpatient services under the proposed hospital outpatient prospective payment system (OPPS) rule issued August 8 by the Centers for Medicare and Medicaid Services (CMS). Full Story
Articles & Analyses
- New IRS Independent Contractor Test, By Sidney S. Welch and Tizgel K. S. Mark, Powell Goldstein LLP
- 2005-2006 Physician Organizations Year In Review, Compiled by Health Lawyers' Physician Organizations Practice Group
- 2005-2006 Regulation, Accreditation, And Payment Year In ReviewCompiled by Health Lawyers' Regulation, Accreditation, And Payment Practice Group
Current Topics
- Antitrust
U.S. Court In Missouri Says Medical Group's Antitrust Suit Against Hospital Failed To Allege Relevant Geographic Market- EMTALA
U.S. Court In Missouri Dismisses EMTALA Claim, Finding No Evidence That Uninsured Patient Was Treated Differently- Food and Drug Law
GlaxoSmithKline Agrees To Pay $70 Million To Settle AWP Dispute- Fraud and Abuse
1. U.S. Court In Nevada Finds No Fact Issue Regarding Whether Government Knew Of FCA Defendant's Billing Practices
2. U.S. Court In Nevada Finds Government Failed To Establish FCA Claims Alleging Physician Improperly Billed Medicare For Tests- Health Policy
Tommy Thompson Issues Paper Urging Medicaid Program Reform- Hospitals and Health Systems
1. Uninsured Settle Pricing Lawsuit With Sutter Health
2. CMS Issues Final Report Outlining Its Strategic Plan For Specialty Hospitals- Long Term Care
1. Sixth Circuit Affirms Civil Money Penalty Imposed On Nursing Home
2. Nursing Home Care Continues To Fall Short, New Report Finds- Managed Care
1. U.S. Court In Maryland Grants Class Certification For Lawsuit Against HMO For Collecting Subrogation Claims
2. U.S. Court In Michigan Finds HMO Had Rational Basis For Denying Out-Of-Network Provider's Reimbursement Claim
3. Kaiser Agrees To Pay $5 Million For Lack Of Oversight Of Transplant Facility- Medicaid
Group Dismisses Lawsuit Seeking To Enjoin D.C. From Enforcing Medicaid
Proof-Of-Citizenship Requirements- Medical Malpractice
New Jersey Supreme Court Says Psychiatrist Can Be Liable For Abandonment Even Absent Duty To Warn- News in Brief
FDA Seeks Comments On UDI System For Medical Devices- Physicians
U.S. Court Of Federal Claims Dismisses Physician's Action Against U.S. Stemming From Loss Of Medical Licenses(c) 2006. Reprinted by permission of AHLA. All rights reserved.
Lawsuit Seeking to Discipline Georgia Physicians for Participation in Executions Dismissed
 From the Federation of State Medical Boards:
From the Federation of State Medical Boards:A lawsuit seeking to require the Georgia Composite State Board of Medical Examiners to punish physicians who participate in executions was dismissed last week by a Fulton County Superior Court judge. Lawyers for seven physicians, including three physicians in Georgia, had sought to have the medical board uphold American Medical Association guidelines that prohibit physicians from involvement in executions.
Lawyers for the state argued that the physicians had no legal standing to sue because they could show no specific harm, and that state law is the determining factor in the administration of lethal injection in Georgia, not the AMA’s ethical guidelines. Complaints were filed with the medical board against a Georgia physician who assisted the department of corrections with executions, but the board declined to discipline him. The lawsuit, filed by a group of Atlanta-area lawyers, unsuccessfully sought to appeal the board’s decision.
In April, Georgia Gov. Sonny Perdue signed a bill (HB 57 [link]) that protects any physician licensed in Georgia from having their license challenged, revoked or suspended if the individual participates, in any way, in the state’s execution process. The Act became effective July 1, 2006, and applies to executions carried out on or after July 1, 2006.
Latest from the law reviews
 From the law library at the University of Washington, here are the latest law journal titles in the health law arena:
From the law library at the University of Washington, here are the latest law journal titles in the health law arena:- Brignati, Michael J. Note. Access to the safe harbor: bioterrorism, influenza, and the Supreme Court's interpretation of the research exemption from patent infringement. (Merck KGaA v. Integra LifeSciences I, Ltd., 125 S. Ct. 2372, 2005.) 13 J. Intell. Prop. L. 375-404 (2006). [L][W]
- Clamon, Joseph B. Does my health insurance cover it? Using evidence-based medicine and binding arbitration techniques to determine what therapies fall under experimental exclusion clauses in health insurance contracts. 54 Drake L. Rev. 473-508 (2006). [L][W]
- Fisher, Breighanne Aileen. Comment. Community-based efforts at reducing America's childhood obesity epidemic: federal lawmakers must weigh in. 55 DePaul L. Rev. 711-743 (2006). [L][W]
- Sulentic, Alison McMorran. Now I lay me down to sleep: work-related sleep deficits and the theology of leisure. 20 Notre Dame J.L. Ethics & Pub. Pol'y 749-783 (2006). [L][W]
- Symposium 2006: The Changing Face of White-Collar Crime. Foreword by Special Sections Editor Russel J. Chibe; articles by Kathleen F. Brickey, John F. Cooney, Stuart H. Deming, Barbara Crutchfield George, Kathleen A. Lacey, Judge James F. Holderman, Charles B. Redfern, Joan H. Krause, William R. McLucas, Howard M. Shapiro, Julie J. Song, Julie R. O'Sullivan and Robert N. Rabecs. 96 J. Crim. L. & Criminology 389-756 (2006). [L][W]
- Dedication [to Steven P. Frankino] Smith, George P., II. A fond farewell. 22 J. Contemp. Health L. & Pol'y 1-4 (2005). [L][W]
- Hodge, James G., Jr., Lance A. Gable and Stephanie H. Calves. The legal framework for meeting surge capacity through the use of volunteer health professionals during public health emergencies and other disasters. 22 J. Contemp. Health L. & Pol'y 5-71 (2005). [L][W]
- Lenz, Connie. Prescribing a legislative response: educators, physicians, and psychotropic medication for children. 22 J. Contemp. Health L. & Pol'y 72-106 (2005). [L][W]
- Rutkow, Lainie. Optional or optimal?: The Medicaid Hospice Benefit at twenty. 22 J. Contemp. Health L. & Pol'y 107-142 (2005). [L][W]
- Hagan, Robert P. Comment. Restaurants, bars and workplaces, lend me your air: smokefree laws as private property exactions--the undiscovered country for Nollan and Dolan. 22 J. Contemp. Health L. & Pol'y 143-176 (2005). [L][W]
- Porter, Brian. Comment. Stopping the practice of authorized generics: Mylan's effort to close the gaping black hole in the Hatch-Waxman Act. 22 J. Contemp. Health L. & Pol'y 177-209 (2005). [L][W]
- Moyse, David. Comment. Urban legend: dispelling the myth that rural hospitals require increased federal funding at the expense of urban hospitals. 22 J. Contemp. Health L. & Pol'y 210-232 (2005). [L][W]
- Blake, Patrick D. Note. Redefining physicians' duties: an argument for eliminating the physician-patient relationship requirement in actions for medical malpractice. 40 Ga. L. Rev. 573-613 (2006). [L][W]
- Carlson, Katie Ervin. Note. A study of the effectiveness of mandated state contraceptive coverage in Iowa and Missouri and the case for a federal law. 54 Drake L. Rev. 509-534 (2006). [L][W]
- Hopwood, Kaycee. Comment. "For It's One, Two, Three Strikes, You're Out ..." 39 J. Marshall L. Rev. 493-514 (2006). [L][W]
"L" and "W" signify links to Lexis/Nexis and WestLaw.
Should prisoners be enrolled in riskier drug studies?
 The New York Times has an interesting article on this question, spurred by a recent report of the Institute of Medicine that recommends altering the "minimal risk" standard that now applies to prisoners as long as the greater risks are accompanied by the potential of some benefit to the prisoners themselves. The IOM's press release on the report and recommendations is here.
The New York Times has an interesting article on this question, spurred by a recent report of the Institute of Medicine that recommends altering the "minimal risk" standard that now applies to prisoners as long as the greater risks are accompanied by the potential of some benefit to the prisoners themselves. The IOM's press release on the report and recommendations is here.
Thursday, August 10, 2006
AHLA Health Lawyers Weekly (04 Aug 2006)

With the permission of the AHLA, here's the TOC for last week's HLW [members only] (which came in while I was on vacation); this week's TOC should be available tomorrow.
- Top Stories
- CMS Issues Final IPPS Rule That Phases-In Move To Cost-Based System
The Centers for Medicare and Medicaid Services (CMS) issued August 1 the
much-anticipated inpatient prospective payment system (IPPS) final rule for
fiscal year (FY) 2007 that seeks to improve the accuracy of hospital payments by moving from a charge to cost-based system and by accounting more fully for patient severity. - CMS, OIG Release Final Health IT Rules
The Centers for Medicare and Medicaid Services (CMS) and the Department of Health and Human Services (DHHS) Office of Inspector General (OIG) released final rules August 1 to speed the adoption of electronic prescribing and electronic health records. - Articles & Analyses
- OIG Publishes Report Of Claims Billed By Independent Diagnostic Testing
Facilities
By Marla P. Spindel, Powers, Pyles, Sutter & Verville, P.C. - 2005-2006 Long Term Care Year In Review
Compiled by Health Lawyers' Long
Term Care Practice Group - 2005-2006 Medical Staff, Credentialing, and Peer Review Year In
Review
Compiled by Health Lawyers' Medical Staff, Credentialing, and Peer
Review Practice Group - Current Topics
- Corporate Governance
- GAO Releases Survey Results Of Nonprofit
Hospitals' Executive Compensation Practices
- GAO Releases Survey Results Of Nonprofit
- ERISA
- Eleventh Circuit Upholds Dismissal Of State Law Claims Against Insurer Of ERISA-Governed Health Plan That Failed To Disclose Lapsed Plan
- Food and Drug Law
- FDA Announces Plans For OTC Marketing Of Plan B
- Enzi, Kennedy Introduce Drug Safety Bill
- Fraud and Abuse
- First Circuit Vacates Three-Month Prison Sentence For Executive Convicted Of Conspiring To Defraud Medicare Of Over $5 Million
- Update
- Health Information Technology
- DHHS Recognizes CCHIT Criteria For EHRs, Releases Certification Guidance Document
- Hospitals and Health Systems
- Grassley, Baucus Question Whether CMS' Specialty Hospital Study Is Flawed
- HSC Study Discusses Implications Of Growth In ASCs And Rising Specialty-Service Competition
- Managed Care
- Florida Appeals Court Overturns Dismissal Of Hospital's Action Seeking Reimbursement For Emergency Services Rendered To HMO Subscriber
- Medical Records
- California Appeals Court Finds Health Plan May Disclose To Its Attorney Medical Records Of Potential Malpractice Complainants
- Medicare
- GAO Sees Promise In CMS' New Cost-Based Approach Under IPPS
- CMS Announces First Contract For Payment Of Medicare Part A And B Claims
- Grassley, Baucus Introduce Bill To Improve Accuracy Of Medicare Payments To Hospitals
- OIG Finds Medicare Part B Drug Costs Would Have Been Cut By $16 Million Had CMS Applied Authorized Price Adjustment
- CMS Issues Final Rule Updating Payment Rates For IRFs In FY 2007
- CMS Issues Final Rule On Accreditation Of DME Suppliers
- CBO Researcher Says Medicare Spending Per Beneficiary Has Slowed In Recent Years
- OIG Releases Reports On Medicare Beneficiary Access To Home Health Agencies And Skilled Nursing Facilities
- Report Says Medicare Beneficiaries With HIV/AIDS Face High Out-Of-Pocket Costs Under Part D
- News in Brief
- JCAHO Will Test Measures For Inpatient Psychiatric Services
- NCQA Adds Two New Standards To Its 2007 Accreditation Standards For Managed Care Plans
- Physicians
- New York Court Finds Physician Not Required To Exhaust Administrative Remedies To Sue For Contract Breach
- Quality of Care
- Report Makes Recommendations For Improvement Of Current Flawed Healthcare System
Wednesday, August 09, 2006
FDA, Barr Pharmaceuticals, reach accord over Plan B contraceptive sales
 In Wednesday's paper, the NY Times reports that the FDA and the manufacturer of the Plan B contraceptive have reached an agreement that may lead to OTC sales (at least to customers 18 years of age and up; under-18's will still need a prescription) within weeks. The story doesn't refer to an earlier report that the FDA offered the deal to Barr last week, on the eve of hearings on the nomination of acting Commissioner Andrew C. von Eschenbach, apparently in response to the announced opposition of Sen. Hillary Clinton and other Senate Dems, who had vowed to delay a vote on the nomination until the FDA cleared its apparently unprecedented derailing of the OTC application. Pro-life groups regard the drug as an abortifacient, despite FDA's official classification of Barr's Plan B drug as an emergency contraceptive, so we can expect this latest development to produce the usual ripple effect of abortion politics.
In Wednesday's paper, the NY Times reports that the FDA and the manufacturer of the Plan B contraceptive have reached an agreement that may lead to OTC sales (at least to customers 18 years of age and up; under-18's will still need a prescription) within weeks. The story doesn't refer to an earlier report that the FDA offered the deal to Barr last week, on the eve of hearings on the nomination of acting Commissioner Andrew C. von Eschenbach, apparently in response to the announced opposition of Sen. Hillary Clinton and other Senate Dems, who had vowed to delay a vote on the nomination until the FDA cleared its apparently unprecedented derailing of the OTC application. Pro-life groups regard the drug as an abortifacient, despite FDA's official classification of Barr's Plan B drug as an emergency contraceptive, so we can expect this latest development to produce the usual ripple effect of abortion politics.
Thursday, August 03, 2006
NH's medical board agrees: doc has 1st Amendment right to be a jerk
 As reported here earlier, a local NH court ruled that Terry Bennett's rude and crude comments to his patients were protected by the First Amendment and couldn't be the basis of a disciplinary case against the doctor. Apparently the New Hampshire State Board of Medicine agrees. As reported by Modern Healthcare today, the Board
As reported here earlier, a local NH court ruled that Terry Bennett's rude and crude comments to his patients were protected by the First Amendment and couldn't be the basis of a disciplinary case against the doctor. Apparently the New Hampshire State Board of Medicine agrees. As reported by Modern Healthcare today, the BoardThis has to be the right result, from a constitutional perspective. Too bad it will probably only encourage the good doctor in his boorish and racist comments. The AP story is here (courtesy of the Boston Globe).will not appeal a court decision that blocked a disciplinary case over comments made by Rochester, N.H., physician Terry Bennett to patients in his private practice. The board sought to determine whether Bennett's comments, which at least three patients characterized as offensive, breached professional ethics standards.


