
Tuesday, July 27, 2004
Medical error: does it kill 195,000 annually?
Monday, July 26, 2004
Health care reform redux.
The NCHC's report, "Building A Better Health System," is worth a read. As the Executive Summary makes clear, the Coalition has done a good job of identifying the most glaring needs of the health care system. What remains to be seen is whether they -- or anyone else -- have a plan to implement the group's five specifications:A broad coalition of insurers, consumers, providers, labor unions, and other groups warned today that absent a dramatic overhaul of the health system in the near future, premiums will soar and the number of uninsured will rise sharply. Soaring health costs are reducing economic growth and new jobs, corporate profits, the competitiveness of corporations, and the viability of pensions, said the National Coalition on Health Care. . . .
The coalition said that if policymakers fail to act, premiums for family coverage will top $14,500 in 2006, and the number of uninsured will reach 51 million that year. In addition, the deficit will rise by trillions of dollars in coming decades, it added. The coalition calls for legislation covering all Americans within two or three years of enactment. Health cost increases should be brought in line with cost increases in other parts of the economy within five years. "Today's report is politically significant because it shows that there is broad support across most sectors of the economy and society, and across party lines for tough, system-wide reform," said coalition co-chair Paul Rogers, former Democratic House member from Florida. The coalition's honorary co-chairs are former Presidents Clinton, Bush, and Carter.
1. Health Care Coverage for All
• to be achieved within two to three years after the passage of legislation
• defined core benefit package
• employers and individuals able to purchase supplemental coverage beyond core package
• options for insuring all Americans include
- employer mandates (supplemented with individual mandates as necessary)
- expansion of existing public programs that cover subsets of the uninsured
- creation of new programs targeted at subsets of the uninsured
- establishment of a universal publicly financed program
• mandatory participation
• subsidies for less affluent
2. Cost Management
• within five years, bring increases in the costs and premiums associated with the core benefit package into alignment with increases in per capita gross domestic product
• increase the value for patients that is generated by any given level of health care spending
• measures include:
- providing more and better information for patients, providers, and purchasers
- improving quality and outcomes of care and reducing amount of unnecessary and injurious care
- building national information technology infrastructure for health care
- modernizing and simplifying administration
• urgent need for cost relief requires short-term constraints:
- rates for reimbursing providers for care encompassed by core benefit package and
- only after those rates take effect, limits on increases in insurance premiums for coverage defined by that package
• to facilitate comparisons, insurers required to set premiums separately for core benefit package and supplemental coverage
3. Improvement of Health Care Quality and Safety
• accelerated development of an integrated national information technology infrastructure for health care, including:
- protocols for electronic patient records, prescription ordering, and billing
- standards to protect privacy
- mechanisms to incentivize and provide capital for the upfront investments necessary to build the infrastructure
• measures of process and outcomes quality to improve accountability and help payers and patients make better choices
• independent national board, with members drawn equally from public and private sectors, to coordinate public and private efforts to improve quality of care
• board also responsible for coordinating development of national practice guidelines
- guidelines to be based on reviews, by panels of leading health care professionals, of research on impacts of technologies and procedures
- guidelines could be cited in malpractice cases as evidence of best medical practice
• board to update core benefit package to reflect changes in practice guidelines
4. Equitable Financing
• measures to reduce or eliminate cost-shifting across categories of insurance programs and payers
• mechanisms or sources that could be used, individually or in combination, to fund program costs include:
- general revenues
- earmarked taxes and/or fees
- contributions required from employers
- contributions required from individuals and families
• financial obligations to be adjusted, or subsidies provided, based on relative ability to pay for less affluent individuals, families, and employers
5. Simplified Administration
• assurance of coverage for all Americans and establishment of core benefit package to create consistent set of ground rules and reduce variations that now draw time and resources away from protection and advancement of health
• creation of an integrated national information technology infrastructure – including electronic patient records, prescription ordering, and billing – to reduce administrative complexity, costs, and medical errors
• national practice guidelines to reduce variability of care and billing and improve quality of care
The Coalition can't be faulted for failing to think big. Specifically, its report sets out three premises that underlay their detailed specifications:
Health care reform must be a national priority.
Comprehensive health care reform is long overdue. Every year that reform is delayed, tens of millions of Americans live in peril, without health insurance; millions are harmed, and hundreds of thousands die needlessly, because of sub-standard care; and health care costs continue to spiral ever upwards.The Coalition’s specifications are meant not just to encourage and help to frame a national debate about health care reform, but to create momentum for the passage of legislation. These specifications are an expression of operational intent: Our member organizations are determined to work with other organizations and with policymakers in both parties to secure the reforms described here. Yes, we need a vigorous debate about health care policy — but what we really need is action, and soon.
Health care reform must be systemic.
The Coalition’s specifications were developed not as a shopping list of potential stand-alone initiatives, but as a linked series of targets, criteria, and options — meant to be adopted concurrently and to work together. The vast American health care sector is exquisitely and elaborately interconnected. Partial or piecemeal reforms, even those conceived and implemented with the best of intentions, can produce unanticipated adverse consequences far from the focus or locus of those targeted reforms.For example, a dramatic expansion of access, implemented without accompanying measures to improve quality and manage costs, could produce an overloaded health care system that delivers worse care (albeit to more people) at higher costs. Similarly, constraints on costs (and reimbursements for care), pursued in isolation,
could compromise both access and quality.A system is a set of institutions and processes that function together to achieve defined objectives. The Coalition’s specifications were designed to serve multiple goals simultaneously. We began our development of recommendations by agreeing on five core principles for reform (which appear below as section headings for our specifications). Then, as our deliberations proceeded, we continuously revisited and recalibrated our recommendations to make sure that the individual components fused together into a sensible systemic strategy.
We believe that a systemic approach can increase not only the substantive
coherence of reform, but also its political feasibility. Thus, if constraints on health care cost increases were proposed in isolation, providers might understandably anticipate that their revenues going forward would be diminished. By contrast, if those same constraints were conjoined in a systemic strategy with an assurance of
coverage for all Americans and financing for their care, providers would receive payment for care that they now provide, with little or no compensation, to uninsured patients.Health care reform must be system-wide.
The Coalition is calling for system-wide reforms, not for changes that would apply to only some payers, patients, or providers. Unless reform is system-wide, gains in some sectors or for some groups are likely to be offset by losses elsewhere. There is, in addition to this practical consideration, another compelling argument for making certain that reform is system-wide. America is already a nation of health care haves and have-nots. Reform should aim to assure that all Americans receive excellent
health care and are able to enjoy the quality of life and peace of mind for which such care is essential. Piecemeal reform that helps some categories of people to the detriment of others would not take us closer to an optimal health care system and could actually make it harder to attain.
Patient safety, quality bill heads to conference
The Senate approved July 22 legislation intended to improve patient safety by promoting medical error reporting (Congressional Record). The "Patient Safety and Quality Improvement Act" would encourage voluntary error reporting by protecting patient safety data from disclosure so that healthcare providers could report medical errors without fear of being sued. The Senate Committee on Health, Education, Labor and Pensions unanimously approved S. 720 last year, while the House passed a similar bill in March 2003 (H.R. 663). [In parliamentary terms, the Senate incorporated S.720 in H.R. 663, as an amendment and then passed H.R. 663. So now the two versions of H.R. 663 go to conference to be reconciled.] The legislation was prompted by a 1999 Institute of Medicine Report [To Err Is Human] that found as many as 98,000 people die each year as a result of preventable medical errors.
Sunday, July 25, 2004
FDA, preemption, & tort reform.
Preemption starts off easy: Under the Supremacy Clause, federal laws take precedence over state laws. When a state law is inconsistent with federal law (whether in statutes, treaties, court decisions, or the Constitution), the state law gives way to the federal.
That may be the only easy thing about the doctrine. Figuring out what counts as a federal/state conflict, and then determining how broadly preemptive the federal law should be, can be very tricky.
Case in point: the federal laws that regulate drug and medical devices. Should the fact that a manufacturer has jumped through the hurdles of the federal Food, Drug and Cosmetic Act ("FDCA") to prove the safety and efficacy of its drug or device mean that an injured plaintiff cannot sue the manufacturer under state tort law, on the theory that "regulation" of these manufacturers by state tort law should be deemed preempted by the federal statutory regulation of drugs and devices that is already in place? With devices, it's particularly tricky, because medical devices are assisgned to "classes" based upon their potential harm to the user. Class I devices present the least potential harm and are subject to the least regulatory control. Class III devices, on the other hand, go through the most exacting level of agency regulation. When it comes to preemption of state tort law, different levels of regulation might very well be viewed in a different light and produce different results.
In 1996, the Supreme Court had an opportunity to consider the preemptive effect of federal law in a Class III device case. In Medtronic v. Lohr, the Court had to figure out the preemptive effect of language added to the FDCA by the Medical Device Amendments of 1976:
Except as provided in subsection (b) of this section, no State or political subdivision of a State may establish or continue in effect with respect to a device intended for human use any requirement (1) which is different from, or in addition to, any requirement applicable under this chapter to the device, and (2) which relates to the safety or effectiveness of the device or to any other matter included in a requirement applicable to the device under this chapter.The Court concluded that -- despite the relative rigor with which Class III devices are regulated -- the federal statutory language did not preempt state-law claims for negligent design, negligent manufacture, and failure to warn. Primary among the the Court's reasons was the absence of any federal cause of action for injured consumers, so that acceptance of the manufacturers' argument that state law claims are preempted would have left plaintiffs with no remedy whatsoever. It was also important to the Court that with respect to the device involved in this case, Medtronic did not actually go through the high hurdles of Class III regulation, because it successfully claimed that its device was "substantially equivalent" to other peviously approved devices.
Since 1996, the FDA has taken the position that federal law is not broadly preemptive when it comes to state laws that would hold manufacturers to a higher standard than the federal one -- in effect arguing that federal law sets a floor when it comes to design, manufacturing practices, and labeling, but not a ceiling. Starting in 2002, however, the Bush Administration has taken the position that federal law is broadly preemptive and sets both a floor and a ceiling. The result of this shift can be seen in opinions like the Third Circuit's last Tuesday in Horn v. Thoratec Corp. (No. 02-4597), in which the court -- relying heavily on a brief filed by the Bush Administration as friend of the court -- concluded that Medtronic was not controlling precedent and the plaintiff's state tort claims were preempted by the federal law. The principal reason for dissing Medtronic was the fact that the device in the Horn case had in fact gone through the rigorous pre-market approval process for Class III devices rather than rely on the generic requirements that apply to devices that seek marketing approval under the "substantial equivalence" test.
Although this distinction appears to have been on the Supreme Court's mind in Medtronic and has much to recommend itself as a matter of statutory text and regulatory policy, its broad application to claims such as Mrs. Horn's (and numerous other plaintiffs who have been bounced out of court by the Bush Administration's zealous advocacy on behalf of manufacturers) represents a new chapter in the Bush Administration's pursuit of "tort reform," a chapter that leaves many injured patients and their survivors with no relief at all. "Compassionate conservatism"? Hah! Meanwhile, you can read about the shift and its political implications in a good article by Robert Pear in today's New York Times.
Saturday, July 24, 2004
Death penalty & psychotropic drugs.
I have two questions: (1) What do you suppose the U.S. Court of Appeals for the Fifth Circuit will do with this on appeal? (2) What did the physicians who ordered the daily doses of Haldol and Perphenazine think they were doing?
Friday, July 23, 2004
Drug reimportation: video conference.
Unfortunately, the slides presented by Tom McGinnis, Director of Pharmacy Affairs, FDA, aren't included on the Kaiser web site (above) or the Alliance's web site (though the Alliance site has a wealth of supplemental materials). The slides may eventually show up on the FDA web site, but until then, there's the July 14 testimony of William K. Hubbard, Associate Commissioner for Policy and Planning, FDA, before the Senate Judiciary Committee.
FTC/DOJ: Abolish CON laws.
The DOJ/FTC report was issued today and can be found here.
Long-term care: crisis.
Other recent coverage of this issue gives a glimpse into the looming crisis: "Long-Term Care Tests Governors" (Olympia (Wash.) Olympian) . . . "Congressional Briefing on Long-Term Care Alternatives" (U.S. Newsire) . . . Deborah Stone: "Shopping for Long-Term Care" (Health Affairs) . . .
Wednesday, July 21, 2004
JCAHO hit in GAO report for CMS.
As the Associated Press reports today (courtesy of the Indianapolis Star), the Government Accountability Office (GAO (formerly the "General Accounting Office")) has filed a report that is extremely critical of the performance of the Joint Commission for Accreditation of Healthcare Organizations (JCAHO (formerly the Joint Commission for the Accreditation of Hospitals)) in its role as the designated Medicare accreditation-surveyor for the Centers for Medicare and Medicaid Services (CMS (formerly the Health Care Financing Administration). (The link to the GAO report is probably not a stable address; if it stops working, try here.)
The report includes the following:
JCAHO’s pre-2004 hospital accreditation process did not identify most of the hospitals found by state survey agencies in CMS’s annual validation survey sample to have deficiencies in Medicare requirements. In comparing the results of the two surveys, CMS considered whether it was reasonable to conclude that the deficiencies found by state survey agencies existed at the time JCAHO surveyed the hospital. In a sample of 500 JCAHO-accredited hospitals, state agency validation surveys conducted in fiscal years 2000 through 2002 identified 31 percent (157 hospitals) with deficiencies in Medicare requirements. Of these 157 hospitals, JCAHO did not identify 78 percent (123 hospitals) as having deficiencies in Medicare requirements. For the same validation survey sample, JCAHO also did not identify the majority -- about 69 percent -- of deficiencies in Medicare requirements found by state agencies. Importantly, the number of deficiencies found by validation surveys represents 2 percent of the 11,000 Medicare requirements surveyed by state agencies in the sample during this time period. At the same time, a single deficiency in a Medicare requirement can limit the hospital’s capability to provide adequate care and ensure patient safety and health. Inadequacies in nursing practices or deficiencies in a hospital’s physical environment, which includes fire safety, are examples of deficiencies in Medicare requirements that could endanger multiple patients.The AP story provides a little more detail:
The potential of JCAHO’s new hospital accreditation process to improve the detection of deficiencies in Medicare requirements is unknown because the process was just implemented in January 2004. JCAHO plans to move from using announced to unannounced surveys in 2006, which would afford JCAHO the opportunity to observe hospitals’ operations when the hospitals have not prepared in advance to be surveyed. In addition, the pilot test of the new accreditation process was of limited value in predicting whether it will be an improvement over the pre-2004 process in detecting deficiencies. Limitations in the pilot test included that hospitals were not randomly selected to participate; that observers from JCAHO accompanied each surveyor, thus possibly affecting surveyors’ actions; and that JCAHO evaluated the results instead of an independent entity.
CMS has limited oversight authority over JCAHO’s hospital accreditation program because the program’s unique legal status effectively prevents CMS from taking actions that it has the authority to take with other health care accreditation programs to ensure satisfactory performance. For example, requiring JCAHO’s hospital accreditation program to submit to a direct review process or placing the program on probation while monitoring its performance. Further, CMS relies on a measure to evaluate how well JCAHO’s hospital accreditation program detects deficiencies in Medicare requirements that provides limited information and can mask problems with program performance, uses statistical methods that are insufficient to assess JCAHO’s performance, and has reduced the number of validation surveys it conducts.
Many of the overlooked problems related to fire safety, while others involved substandard care.Predictably, JCAHO isn't pleased with the report:
In a Texas hospital, a patient died after receiving a double dose of narcotics in the emergency room. A California hospital lacked "a sanitary environment to avoid sources and transmission of infections and communicable diseases and failed to develop a system for ensuring the sterilization of medical instruments," the report said.
Commission President Dennis O'Leary said his group made sweeping changes to the accreditation process earlier this year.What the AP story doesn't say (and I can't find it in the GAO report, either) is that in the 20+ years JCAHO has been in the "deeming" business with CMS, a few provisionally accredited hospitals have been denied full accreditation, but no fully accredited hospital has ever lost its accreditation. (If any reader can cite me an example that disproves this statement, I'd love to see it.) Long viewed as a toothless tiger by many in the industry, JCAHO ought to feel the sting of this GAO report. And, as denials go, President O'Leary's face-saving comment that "we're better than that now" isn't much of one.
"In our view, it is irresponsible to alarm the public using statistics that have little meaning," O'Leary said in response to the GAO report.
Genetic screening and . . .
Tuesday, July 20, 2004
Drug reimportation.
Blame the lawyers.
Public concern about medical, surgical and diagnostic errors is high and many Americans have doubts about the ability of medical institutions to prevent these types of errors, according to the latest WSJ Online/Harris Interactive health poll.
Sixty-three percent of those polled say they are "extremely concerned" or "very concerned" about medication errors, such as receiving the wrong medicine, that can take place in a hospital. And more than half of respondents are extremely or very concerned about surgical errors.And the source of this problem? According to Vice-President Cheney (as reported by Ceci Connolly in today's Washington Post, and brought to my attention by former student Jonathan Childers):
"This problem doesn't start in the waiting room," Cheney said in remarks released by the campaign. "It doesn't start in the operating room. The problem starts in the courtroom."Here's the quote you will hear over and over again from the Administration between now and the November election: "When it comes to the legal crisis in American health care, the Kerry-Edwards ticket is on the side of personal-injury trial lawyers, and the Bush-Cheney ticket is on the side of doctors and patients." Easy on the ears, easy on the brain. Sure beats having an actual plan to deal with this country's health care problems, doesn't it?
With lawsuits on the rise and multimillion-dollar awards making headlines, physicians and many Republicans say limiting damages is the solution to the broader challenges confronting the U.S. health system. In their analysis, capping damages will lead to lower malpractice premiums, which will reduce doctors' use of unnecessary tests and procedures, known as defensive medicine. Those improvements will result in better care at a lower cost, enabling more people to buy coverage, they say.
Monday, July 19, 2004
Class actions suits against non-profit hospitals.
- Several high-profile law firms have filed 31 cases against non-profit hospitals and hospital chains since late June. The cases make similar allegations, including: Some hospitals violate an implicit contract with the government to provide charity care in exchange for tax-exempt status by charging uninsured patients more than the insured or aggressively pursuing debts from low-income patients.
- Some facilities have large cash reserves they should use to provide charity care.
- They allow for-profit entities, such as doctor groups, to use their facilities to earn a profit.
- Hospitals named include the Cleveland Clinic; New York-Presbyterian; Sutter Health in Sacramento; Advocate Health Care Network in Chicago; Phoebe Putney Health Systems in Albany, Ga.; Baptist Health Systems in Alabama; and Catholic Healthcare Partners in Cincinnati.
Patient advocates, who were the first to publicize some of the concerns now included in Scruggs' cases, have mixed thoughts on the lawsuits. "This is a huge wake-up call," says Claudia Lennhoff of the Champaign County Health Care Consumers group in Illinois.
They also worry about the financial effect of the cases on non-profits.
"Having more scrutiny of billing practices is a good thing, but the risk is we're not taking on big tobacco, we're taking on a vital service," says Mark Rukavina of the Access Project, a national resource center that works with local groups on health care issues. "It's an industry I want to preserve, not bring down."
Some health law attorneys are skeptical that Scruggs' arguments will succeed.
"The behaviors they're targeting (billing and collection practices against the uninsured) are atrocious in some circumstances, but they're not illegal," says Gregg Bloche, a law professor of health law at Georgetown University. "The suits will fail."
Nor do they think there is an implied contract between hospitals and the government.
"That's never been recognized in the law," says Stuart Gerson, a partner at Epstein Becker & Green in Washington, D.C., who represents a hospital being sued. "The idea of an individual citizen, a taxpayer, seeking to enforce charitable obligations is, at least, a very novel argument that finds little support."
If any laws are being broken by the common hospital practice of allowing for-profit doctors to use their facilities, or if facilities are improperly steering business to trustees' companies, those arguments should be heard by taxing authorities or federal and state antitrust or anti-kickback regulators, Gerson says.
The lawsuits are renewing debate over the legal and ethical responsibility the nation's non-profits have to provide charity care.
"The IRS has never been really clear about what the grant of tax-exempt status means," says attorney John Reiss of the law firm Saul Ewing in Philadelphia. "It's never been clear that it actually commits you to providing any particular amount of charity care or anything else."
Non-profit hospitals say they provide a variety of charitable services. Hospitals have different ways of classifying such care, with some saying charity is providing medical services to anyone who walks in the ER, regardless of their ability to pay.
Others consider write-offs for bad debt charity care or financing community services, such as supporting health clinics.
Sunday, July 18, 2004
More on designer babies.
The HFEA's possible relaxation of the rules in this field is already stirring up opposition:A two-year-old boy who needs urgent treatment to cure a rare and potentially fatal blood disorder is at the centre of a fresh row over creating "designer babies" with human embryos.
The Human Fertility and Embryology Authority (HFEA) is poised to relax its rules on using genetic screening for medical treatments on Wednesday. The decision will have profound consequences for the life of Joshua Fletcher and children like him.
Joshua suffers from a rare genetic defect called Diamond-Blackfan anaemia, one requiring regular blood transfusions. His parents, Joe and Julie Fletcher, from County Antrim, Northern Ireland, have asked the HFEA to permit the genetic selection of a healthy sibling to help cure him by using that baby to donate healthy stem cells.
The present HFEA rule prevents parents selecting embryos solely because that child will have desirable characteristics, even if they will save another life - the central issue in Joshua's case.
Two years ago, the HFEA was heavily criticised for rejecting a similar bid by the family of Charlie Whitaker, who suffered from the same disorder. His parents instead flew to Chicago for fertility treatment, and had a genetically matched son. . . .
At present, embryos can only be screened before implantation using a technique called pre-implantation genetic diagnosis if there is a significant risk that the baby will itself be born with a critical or extremely serious genetic condition. Using that child to then treat another child is currently seen by the HFEA as a secondary benefit of the technique.
The proposal, which follows an HFEA rule review, has already provoked a fierce controversy over the religious and medical ethics of creating "made to order" babies simply to save another child's life. Critics claim embryo selection could easily lead to parents selecting babies because of their hair colour, gender or intelligence, and eventually to cloning.
Yesterday, a Christian think tank warned that an HFEA rule relaxation would be instantly challenged in the courts. Roger Smith, of the Centre for Bioethics and Public Policy, said: "The law says the welfare and best interests of the child being born has to be their primary consideration - not creating one life for the sake of another. That seems to us to be outside ethical boundaries."
The HFEA is also under intense pressure from fertility experts, parents and medical charities to soften its regulations after rejecting earlier bids by other families with Diamond-Blackfan anaemia. One family flew to the United States for treatment. Three further families with children with Diamond-Blackfan anaemia are preparing bids to the HFEA.
An HFEA spokeswoman said the authority could either reject the Fletchers' proposal or seek further information before making a decision.
Conceiving a child to save another.
An article in today's Arizona Daily Star discusses the practice of conceiving a child in order to produce a donor (bone marrow, cord blood . . . ), which the editors describe as "deeply controversial." In and of itself, it's hard to see where the moral objection, or the argument for regulation, comes in. The article identifies a few problems, only one of which focuses on the decision to conceive for the benefit of another:
- Dr. Michael Graham, director of pediatric bone-marrow transplantation at University Medical Center (in Phoenix): "The underlying principle of medical ethics is that no person can exist solely for somebody else's benefit. So I worried about creating a child specifically to create a donor." The key word in this Kantian objection is solely, which posits that the utilitarian reason for having another child is the only reason, rather than one of many. The fact that a child is born for reasons that benefit others (parents who want the additional companionship in older age, a sibling who would otherwise be an only-child) hardly seems like a reason not to conceive, as long as the child will be valued, loved, and protected in his or her own right.
- Of course, that last notion does give one pause, certainly when you hear about a family that plans to put the "donor baby" up for adoption after the donation has occurred (so far, a hypothetical concern only). Dr. Graham "was especially concerned about a North Carolina mother with a diseased child who took fertility drugs to try to have a 'donor baby,' even though she was divorced. She had twins, but neither turned out to be a match. 'So here are two more children in a split and strained family,' he said." True, but that North Carolina mom could have had babies for any reason, or no reason at all, with the same result. The question is how paternalistic do we want to be.
- In vitro fertilization raises other issues, at least for the Catholic church and others who object to the creation of multiple embryos, followed by genetic screening to identify the best match, and destruction of the unused embryos.
Interestingly, "some ethicists have argued it might be morally wrong not to have a donor or designer baby, if possible, when another child's life is at stake. 'In a situation that requires an intervention involving no sacrifice and no inconvenience by one child in order to save the life of another child, (this) is morally acceptable. It may even be morally required,' Dr. Merle Spriggs, head of the Ethics Unit at Royal Children's Hospital in Victoria, Australia, wrote in a British medical journal."
Saturday, July 17, 2004
Reimportation bill stalls in Senate.
COMPARING THE BILLS
Two U.S. Senate bills would allow drug importation into the United States from Canada and other countries. How they compare: Pharmaceutical Market Access and Drug Safety Act (Democrat-sponsored) [S.2328] 1. The FDA has 90 days to create rules permitting drug importation. U.S. pharmacies and drug wholesalers can import medicines from Canada in the first year and 19 other countries thereafter. Individuals could be shipped prescriptions from mail order or Web sites from FDA-approved Canadian pharmacies. 2. It's unlawful for drugmakers to limit supply or alter drugs to fail FDA standards. 3. A 1 percent user fee is imposed to fund FDA inspections. 4. Exporters to individuals must post a bond that they forfeit if they send unsafe drugs.
Safe Importing of Medical Products and RX Therapies Act (GOP-sponsored) [S.2493] 1. The FDA has one year to make safety recommendations before permitting imports from Canada and up to three years for 15 European Union countries. The FDA could ban drugs from some nations. 2. There is no provision making it unlawful to reduce supply or alter drugs to fail FDA standards. 3. A new, uncapped user fee program is established, paid for by all foreign and domestic businesses engaged in importation to pay for FDA inspections. 4. Licensing requirements and penalties are established for all online pharmacies that illegally conduct or solicit U.S. business.
Gary Hart: no joke.
Few Americans have more right to say ''I told you so'' than Gary Hart. During the 1990's, when the foreign policy establishment was obsessed with Star Wars and other issues left over from the cold war, Hart headed a commission on national security with another former senator, Warren Rudman. Its report, issued early in 2001, warned of catastrophic terrorist attacks in which ''Americans will likely die on American soil, possibly in large numbers.'' Incredibly, the work of the Hart-Rudman commission was widely ignored by the press and the Bush administration.
''The Fourth Power'' builds on the many ideas of the commission, offering sweeping recommendations for how America should orient its foreign policy in the 21st century. Hart's timely central argument -- an alternative to both the neoimperialist impulses of the Bush administration and the creeping Kissingerian realism of the Kerry campaign -- is that the traditional military, political and economic powers of American foreign policy should be constrained by and imbued with a fourth power, America's unique principles. To those who advocate a crusading foreign policy of preemption to ''rid the world of evil'' and spread democracy -- even at the point of a gun -- Hart argues that the first casualty would often be America's moral authority: ''There is a vast difference between advocating, as I do, that America live up to its own principles and advocating, as the Bush administration does, that the rest of the world live up to America's principles.'' At the same time, Hart counters Kerry's retreat to a Kissinger-style foreign policy, based largely on America's interests, with a humble but still idealistic internationalism, with the spread of liberal democracy at its core. It's a call for nation building without Abu Ghraib.
In 1993, Hart sent President Clinton a memo arguing that the end of the cold war was the ideal occasion to reorient the military ''for new missions relating to hostage rescue, counterterrorism, low intensity conflict, guerrilla warfare and stabilization of new democracies.'' Much of this prescient document is reprinted as an appendix. We were told.
Readers respond to PAS column by Kristof.
Wednesday, July 14, 2004
Assisted suicide and Ashcroft.
On Monday, Ashcroft's Justice Department sought reconsideration by the appellate court, which prompted a thoughtful op-ed piece by Nicholas D. Kristof in today's N.Y. Times. Even if you (like me) thought the Oregon law was a bad idea, this is worth reading. I admit that I've come around on this subject because of the Oregon experiment, and Kristof highlights important aspects of that experience quite well.
House votes to allow Canada drug imports.
Federal marriage amendment dies in Senate.
The vote by the Republican-controlled Senate amounted to an embarrassing defeat for President Bush and conservative leaders who had pushed hard for approval of the amendment as a way of protecting traditional marriage. But Senate GOP leaders vowed to continue pushing for the amendment, hoping it will galvanize conservatives in the November election and help elect more supporters of the amendment.The World's Greatest Deliberative Body comes through again!
"This issue is not going away," Majority Leader Bill Frist (R-Tenn.) said.
Tuesday, July 13, 2004
CMS: Lying to Congress.
Regardless of the legal technicalities, it is a terrible policy to deprive legislators of information they need to make informed choices. Mr. Foster has said that he shared his estimates not only with Mr. Scully, but also with Doug Badger, President Bush's health policy adviser. Both Mr. Scully and Mr. Badger declined an invitation to appear before the House Ways and Means Committee in April. The committee should call both men again, under subpoena if necessary, to answer questions about what looks like a conspiracy to keep Congress in the dark.Amen.
Physician recruitment on FBI's radar.
Monday, July 12, 2004
Nonprofits under scrutiny.
Late-term abortion law struck down again.
Sunday, July 11, 2004
Bush's marriage thing.
Science & politics redux.
For years, Advocates for Youth, a Washington-based organization devoted to adolescent sexual health, says, it received government grants without much trouble. Then last year it was subjected to three federal reviews.Sound paranoid? It gets worse: "Professor Parker is also a co-chairman of the International Working Group on Sexuality and Social Policy, an association of researchers and other professionals, which released a report two weeks ago citing examples of what it called sex policing under the Bush administration. The report cited, for example, changes in factual information about sex education and H.I.V. transmission on government Web sites as well as questioning by members of Congress about research grants approved by the National Institutes of Health."
James Wagoner, the president of Advocates for Youth, said the reviews were prompted by concerns among some members of Congress that his group was using public funds to lobby against programs that promoted sexual abstinence before marriage. Although that was not the case, Mr. Wagoner said, the government officials made their point.
"For 20 years, it was about health and science, and now we have a political ideological approach," he said. "Never have we experienced a climate of intimidation and censorship as we have today."
Mr. Wagoner is among the professionals in sex-related fields who have started speaking out against what they say is growing interference from conservatives in and out of government with their work in research, education and disease prevention.
A result, these professionals say, has been reduced financing for some programs and an overall chilling effect on the field, with college professors avoiding certain topics in their human sexuality classes and researchers steering clear of terms like sex workers in the title of grant applications for fear of drawing attention to themselves.
"Programs almost have to hide what they do," said Richard Parker, a professor at the Mailman School of Public Health at Columbia University. "We have a major challenge ahead of ourselves."
Saturday, July 10, 2004
Stem cells and cloning.
Mixing science & politics (again).
Office of Global Public Health will choose which, if any, US Government scientists can serve as advisers to WHO. Instead of going directly to the experts they want as technical advisers, as WHO has done in the past, the organisation must now provide the Office of Global and Public Health, which is headed by a political appointee, with "terms of reference" for each proposed consultation--a process that it concedes "will require a minimum lead-time of 3 weeks". A written directive goes on to remind WHO that US Government employees are required "to serve as representatives of the US Government at all times and advocate US Government policies".The editors allow as how HHS' denials ring a little hollow in light of this administration's demonstrated willingness to shade the truth when science doesn't quite fit its political plans. I like the editors' suggestions for ways the Bushites can prove their sincerity:
A spokesman for the HHS strongly denied charges that this newly resurrected policy represents any attempt by the Bush administration to exercise political control over the exchange of scientific information, describing it instead as a method "to create accountability" and to ensure that WHO works with appropriate experts. He said that agency heads have not always been aware of the consulting activities of their employees, and that no specific cases prompted the action.
But let us give the Bush administration the benefit of the doubt. If this move is meant to provide accountability, let us have some from HHS. First, to dispel any perception of divided loyalties, put a career civil servant, not a political appointee, in charge of the process. Then, to ensure proper public accountability, let HSS put on its website names of those consultants WHO asked for, whether HHS agreed with the requests or approved someone else, and the rationale for the decision. Finally, disclose all of the evidence: how long did the approval process take, and at what cost? The public, whom the administration claims to be protecting, can then decide whether this policy streamlines or obfuscates the process of global scientific consultation, and whether it is a good use of the government's time--and taxpayers' money. We would guess not.
2 new cases from Texas Supreme Court.
Mid-Cities Surgi-Center employed a scrub technician who stole fentanyl, an anesthetic, from the surgical center. Apparently using the same syringe, the technician removed fentanyl from the glass ampules in which it was stored, injected himself with the drug, then injected saline solution back into the ampules to hide his theft. He then re-sealed the ampules with super glue and re-wrapped them with cellophane to further hide his crime. Because the technician was infected with Hepatitis C, his use of a dirty syringe allegedly contaminated the ampules. A number of patients who received fentanyl injections, including the four plaintiffs in this lawsuit, subsequently tested positive for Hepatitis C. Plaintiffs sued Mid-Cities Anesthesiology, P.A., a professional association of ten doctors who practiced anesthesia at the surgical center, and the association's member anesthesiologists. The patients alleged numerous negligent actions against the doctors' association and its members, including negligence in "failing to properly secure anesthesia narcotics" and in "exposing patients to contaminated medication." The association's professional liability insurer originally assumed defense of the suit, but later became insolvent. The Texas Property and Casualty Insurance Guaranty Association (TPCIGA) then assumed its obligations.
TPCIGA tendered the suit for a defense and coverage to the Association's general liability insurer at the time of the litigation, American Indemnity, which denied coverage because it was not the insurer at the time the infections occurred. TPCIGA then tendered the suit to the Association's general liability insurer at the time the plaintiff's became infected, Utica National, which denied coverage based upon an exclusion in its policy for "[b]odily injury . . . due to rendering or failure to render any professional service." After TPCIGA and American Indemnity settled the claims, they brought suit against Utica National for defense and settlement costs. The trial court granted motions for summary judgment by TPCIGA and American Indemnity, holding that Utica National's exclusion for professional services did not preclude coverage and awarded judgment against the defendant for the defense costs and full settlement costs, with attorney's fees and pre- and post-judgment interest. The Court of Appeals in Austin affirmed.
Held: Reversed in part and remanded. The policy excludes coverage only when the insured has breached the standard of care in rendering those professional services. In this case, the allegations in the pleadings raised both the possibility that the treating doctors were negligent in their administration of the drug and the possibility that the doctors' association was negligent in the storage of that drug. Because the plaintiffs alleged both professional and non-professional negligence, the general liability insurer had a duty to defend the underlying suit in this case under the eight-corners doctrine. But because a fact issue exists about whether the patients' injuries were caused at least in part by the doctors' rendition of professional services, in which event Utica National's policy would not cover the doctors' association, the Supreme Court remanded the indemnity claims to the trial court for further proceedings.
Justice Hecht dissented in an opinion joined by Justice Owen. The essence of his opinion is set out in the following passage: "I cannot see how it is remotely possible for a physician to be negligent in preserving the purity of medications administered to patients by himself and those with whom he associates and yet not be in breach of a professional standard of care. Thus, I would hold that the patients' claims were for professional liability, against which Utica had no obligation under its CGL policy to defend or indemnify. The Court does not foreclose this result but remands for fact findings. If I am correct C if the association and its members could not have been negligent without violating a professional standard of care C the outcome will eventually be the same."
Kaelyn Martinez, age 3, underwent a tonsillectomy at the Val Verde Regional Medical Center. Kaelyn’s parents, Marcus Martinez and Mary Koog, filed suit a little over two years after the operation, individually and on behalf of Kaelyn, against the Val Verde County Hospital District (which operates as the Medical Center) and others. The Hospital District is a governmental unit immune from suit under the Texas Tort Claims Act, Tex. Civ. Rem. & Pract. Code § 101.001 et seq., but Martinez and Koog invoked the Act's exception for liability based upon the use of tangible property,
id. § 101.021(2).
The Act requires that a governmental unit receive notice of any claim against it within six months of the incident giving rise to the claim unless it already has actual notice. Id. § 101.101. The Hospital District first received notice of the claims of Kaelyn and her parents six months and twenty-two days after Kaelyn’s surgery, and Martinez and Koog did not contend that the Hospital District had actual notice before then. Accordingly, the Hospital District filed a plea to the jurisdiction, asserting that because it did not receive notice as
required by the Act, its immunity from suit was not waived, and the court lacked subject matter jurisdiction of the claims against it. The trial court sustained the plea and ordered the case dismissed with prejudice.
The parents argued (1) that the notice requirements of the Act are not jurisdictional and therefore the trial court had subject matter jurisdiction of their claims, and (2) that the notice provision of the Act should be tolled if the claimant is a minor, unless the statute clearly states that its time limits are not tolled during a claimant's minority. The court of appeals held that Kaelyn’s minority did not toll the six-month period for giving notice. The court also ruled that notice is not a condition of the Act’s waiver of immunity that should be raised in a plea to the court's jurisdiction but instead is an affirmative defense that should be raised by motion for summary judgment. The court therefore reversed the trial court’s dismissal for want of jurisdiction and remanded the case for further proceedings.
Held: Affirmed. As to the appellants' tolling argument, the Supreme Court, per Justice Hecht, observed, "One can believe, as the court of appeals did, that it is unfair to require a minor who cannot sue to give the notice required by the Tort Claims Act, but the State is not required to waive immunity from suit at all. The fairness or wisdom of the waiver is not our province to decide."
French ban human cloning.
Thursday, July 08, 2004
Studies Look at Health Care in the U.S.
What may surprise readers, and certainly surprised this writer, is that Americans, by paying so much more, do not have many more services. In fact, according to recent research, they typically have fewer. Consider the number of doctors. In 2001, the United States had 2.7 doctors per 1,000 people, compared with a median of 3.1 in the countries in the Organization for Economic Cooperation and Development. France, accused of having a doctor shortage in last summer's heat wave, had 3.3 per 1,000.
Also, consider the number of hospital beds. The United States has only 2.9 hospital beds per 1,000 people, compared with the O.E.C.D. median of 3.9. Germany has 6.3. The United States is also behind in the actual days spent in a hospital and hospital admissions per capita. These are not necessarily bad in themselves, but the question is why we spend so much.
The reason for the high level of American spending, argue the researchers - Uwe E. Reinhardt of Princeton and Peter S. Hussey and Gerard F. Anderson of Johns Hopkins - is that American doctors and hospitals charge much more. Americans also usually pay significantly more for drugs, they say, and administration expenses are exorbitant.
Wednesday, July 07, 2004
OIG's statement re: Scully, the CMS chief auditor, and the price of Medicare reform.
Also, OIG may have concluded that Scully broke no laws, but apparently the nonpartisan Congressional Research Service concluded otherwise. At least, that's what Rep. Charlie Rangel said in a letter to the chairman of the House Ways and Means Committee, in which he quotes from the CRS report that he (Rangel) requested. So far, at least, the CRS report itself seems not to be available on the Web.
According to Rangel's letter, the laws in question are 5 U.S.C. § 7211, §§ 618 and 620 of P.L. 108-199 [NOTE: see 118 Stat. 354-55 (pp. 352-53 of 455)], 42 U.S.C. § 1317, and 5 U.S.C. § 2302(b)(8).
For a great summary of the issues, the responses, and the next steps for this controversy: Kaiser Family Foundation's Daily Health Policy Report.
Health Affairs' mega-med-mal issue.
- The Forgotten Third: Liability Insurance And The Medical Malpractice Crisis, William M. Sage [Abstract]:
- Although the most visible manifestations of medical malpractice involve patient safety and the legal process, the availability and affordability of liability insurance largely determine the direction of medical malpractice policy. Scientific and industrial developments since the first modern malpractice crisis in the 1970s reveal major problems with the structure and regulation of liability insurance. Comprehensive reforms that approach medical malpractice insurance as a health policy problem are needed, and the Medicare program may have a major role to play.
- A Mediation Skills Model To Manage Disclosure Of Errors And Adverse Events To Patients, Carol B. Liebman and Chris Stern Hyman [Abstract]:
- In 2002 Pennsylvania became the first state to impose on hospitals a statutory duty to notify patients in writing of a serious event. If the disclosure conversations are carefully planned, properly executed, and responsive to patients’ needs, this new requirement creates possible benefits for both patient safety and litigation risk management. This paper describes a model for accomplishing these goals that encourages health care providers to communicate more effectively with patients following an adverse event or medical error, learn from mistakes, respond to the concerns of patients and families after an adverse event, and arrive at a fair and cost-effective resolution of valid claims.
- Improving The Medical Malpractice Litigation Process, Catherine T. Struve
[Abstract]:- Critics charge that judges and juries are incompetent to address medical liability issues. Some advocate shifting authority away from ordinary judges and juries, either by appointing "expert" decisionmakers, such as "medical screening panels" or specialized "medical courts," or by instituting caps on damages. Problems with the tort liability system may weigh in favor of a shift to a no-fault administrative compensation system. If the current fault-based system is retained, however, policymakers should not adopt half-measures by creating "expert" panels or "expert" courts. Rather, they should better equip the existing decisionmakers to deal with liability and damages questions.
- Caring For Patients In A Malpractice Crisis: Physician Satisfaction And Quality Of Care, Michelle M. Mello, David M. Studdert, Catherine M. DesRoches, Jordon Peugh, Kinga Zapert, Troyen A. Brennan, and William M. Sage [Abstract]:
- The rhetoric of malpractice reform is at fever pitch, but political advocacy does not necessarily reflect grassroots opinion. To determine whether the ongoing liability crisis has greatly reduced physicians’ professional satisfaction, we surveyed specialist physicians in Pennsylvania. We found widespread discontent among physicians practicing in high-liability environments, which seems to be compounded by other financial and administrative pressures. Opinion alone should not determine public policy, but physicians’ perceptions matter for two reasons. First, perceptions influence behavior with respect to practice environment and clinical decision making. Second, perceptions influence the physician-patient relationship and the interpersonal quality of care.
- Are Damages Caps Regressive? A Study Of Malpractice Jury Verdicts In California, David M. Studdert, Y. Tony Yang, and Michelle M. Mello [Abstract]:
- Caps on damages have emerged as the most controversial legislative response to the new malpractice crisis. We analyzed a sample of high-end jury verdicts in California that were subjected to the state’s $250,000 cap on noneconomic damages. We found strong evidence that the cap’s fiscal impact was distributed inequitably across different types of injuries. In absolute dollar terms, the reductions imposed on grave injury were seven times larger than those for minor injury; the largest proportional reductions were for injuries that centered on pain and disfigurement. Use of sliding scales of damages instead of or in conjunction with caps would mitigate their adverse impacts on fairness.
Tuesday, July 06, 2004
Scully pressured actuary, didn't break law: OIG reports
Monday, July 05, 2004
Do pediatricians need lawyers in order to provide good care?
PEDIATRICS Vol. 114 No. 1 July 2004, pp. 224-228
--------------------------------------------------------------------------------
SPECIAL ARTICLE
Why Pediatricians Need Lawyers to Keep Children Healthy
Pediatricians recognize that social and nonmedical factors influence child health and that there are many government programs and laws designed to provide for children’s basic needs. However, gaps in implementation result in denials of services, leading to preventable poor health outcomes. Physician advocacy in these arenas is often limited by lack of knowledge, experience, and resources to intervene. The incorporation of on-site lawyers into the health care team facilitates the provision of crucial legal services to vulnerable families. Although social workers and case managers play a critical role in assessing family stability and finding appropriate resources for families, lawyers are trained to identify violations of rights and to take the appropriate legal steps to hold agencies, landlords, schools, and others accountable on behalf of families. The incorporation of lawyers in the clinical setting originated at an urban academic medical center and is being replicated at >30 sites across the country. Lawyers can help enhance a culture of advocacy in pediatrics by providing direct legal assistance and case consultation for providers, as well as jointly addressing systemic issues affecting children and families. Until laws to promote health and safety are consistently applied and enforced, pediatricians will need lawyers to effectively care for vulnerable children.
--------------------------------------------------------------------------------
Barry Zuckerman, MD, Megan Sandel, MD, MPH, Lauren Smith, MD, MPH and Ellen Lawton, JD
From the Department of Pediatrics, Boston Medical Center/Boston University School of Medicine, Boston, Massachusetts
Medicine and literature.
- Moacry Scliar
- Martin Winckler
- Richard Selzer
- Jerome Groopman
- Perri Klass
- John Murray
- Phil Whitaker
- Jed Mercurio
- Khaled Hosseini
- Samuel Shem
- Atul Gawande
- Rita Charon
- Cecil Helman
- Jonathan Kaplan
I hope students in the Law, Literature & Medicine class I teach with Patty Hicks will get the chance to read a few of these.
Meanwhile, permit me this idle speculation: Where have all the serious lawyer-writers gone? Most seem to have settled for the easy bucks of mass-market pot-boilers with an option on the screenplay, soon to be a major motion picture opening in theater near you. Scott Turow is the exception - a gifted writer whose mysteries transcend the genre by lifting the entire enterprise up a level or two. My Scottish compadre, Sandy McCall-Smith, has done the same with his successful series on Mma. Precious Ramotswe of Botswana and her No. 1 Ladies Detective Agency. For another generation, Louis Auchincloss was comfortable writing serious fiction, and his near-contemporary was the poet Archibald MacLeish. And . . . ?
Health reform and the presidential election.
The Vice-President's physician.
Mayer does a good job detailing the extent of the physicians' purchases and, inferentially, his impairment:
According to pharmacy records and customer invoices, in July, 2000, for example, the month that Malakoff wrote the letter certifying Cheney’s good health, he purchased thirty bottles of a synthetic narcotic nasal spray called Stadol from two mail-order drug-supply companies. Stadol, which can be addictive, is ordinarily used to treat migraine headaches. Each bottle contains an estimated fifteen doses. In the previous two months, he had bought eighteen bottles. In August, he bought twenty-eight more bottles. During the two-and-half-year period ending in December, 2001, Malakoff spent at least $46,238 online on Stadol and such medications as Xanax, Tylenol with codeine, and Ambien.That's 76 bottles (and 1140 doses) in 5 months. The guidelines for prescribing the drug call for 1 dose, followed by another dose in 60-90 minutes if there is no relief from the first dose, followed by additional 2-dose sequences as needed every 3-4 hours, so Cheney's doc's 7.5 doses per day (assuming he consumed all 1140 doses during the five-month period that he placed his orders) are within the prescription guidelines for the drug. Adding Schedule III-IV drugs like Xanax, Tylenol with codeine, and Ambien, however, suggest a serious problem. (It's unclear, though, how much of the other drugs he was taking. At $92 a bottle (from drugstore.com) he would have spent $41,400 on Stadol during the two-and-a-half-year period described in Mayer's article, leaving only about $5,000 for other drug purchases.)
The issue in all this isn't the fall of a presumably talented physician into the clutches of a dastardly affliction, tragic as that is. The questions raised by Mayer's article, explicitly or implicitly, are:
- Whether the GWU administration responded appropriately when they learned of their colleague's addiction. Even though prescribing under another physician's name and DEA number, which is apparently how these prescriptions were obtained, is a civil and cirminal offense, it appears that GWU informed neither the DC licensing board nor the DEA.
- In addition to the public's legitimate interest in the health of high-ranking public officials, does the public have a similar interest in the health of the health providers who care for those public officials and pronounce them fit for office?
Sunday, July 04, 2004
Medical ethicist: Honesty isn't always the best policy.
Sokol's short piece doesn't provide much guidance for one of the more perplexing debates in medical ethics: whether it's ever ethical to prescribe a placebo for a therapeutic purpose. (Good bibliography here.) Conventional wisdom has it that the placebo effect is lost when the patient is told that she is getting a sugar pill or other inert substance, so the efficacy of the placebo depends upon deception. Sokol allows for the deception when necessary to avoid significant harm ("nonmaleficence"); would his argument also allow for deception in order to achieve a therapeutic benefit ("beneficence")? The overwhelming consensus among ethicists appears to be "no," yet the practice seems to persist among practitioners for what appears to be a variety of reasons. This piece by Gregory Loeben does a nice job of making the case against deception to produce a benefit for the patient.
July 4: George III & George II.
But it is the final sentence of the declaration that deserves the closest study: "And for the support of this Declaration . . . we mutually pledge to each other our Lives, our Fortunes and our sacred Honor." Today, those who believe that the war on terror requires the sacrifice of our liberties like to argue that "the Constitution is not a suicide pact." In a sense, however, the Declaration of Independence was precisely that.
By signing Jefferson's text, the signers of the declaration were putting their lives on the line. England was then the world's greatest military power, against which a bunch of provincial farmers had little chance of prevailing. Benjamin Franklin wasn't kidding around with his quip about hanging together or hanging separately. If the rebel American militias were beaten on the battlefield, their ringleaders could expect to be hanged as traitors.
They signed anyway, thereby stating to the world that there is something worth more than life, and that is liberty. Thanks to their courage, we do not have to risk death to preserve the liberties they bequeathed us. All we have to do is vote.
June cartoon roundup.
 | HAPPY 4th of July |  |
Not a lot on the health care front from our nation's best cartoonists. Maybe that's a good thing, a recognition of the subtle interrelationships among the various seemingly intractable strands of our looming crises of affordability, quality, and coverage for the uninsured. Or maybe it's because Michael Moore and VP Cheney provided such attractive targets . . . .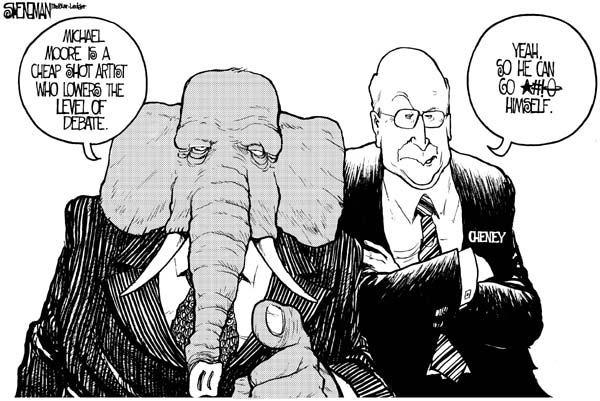
Drew Sheneman, Newark Star-Ledger
As usual, Tom Toles scores a bulls-eye on the Veep: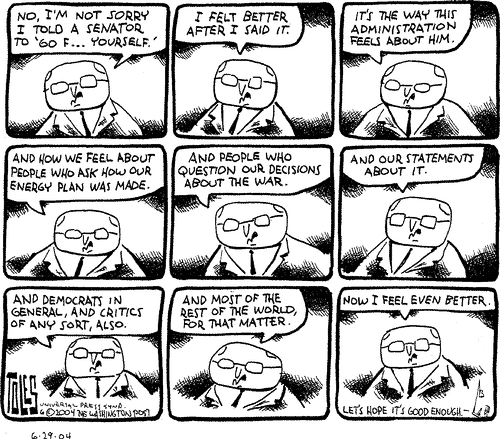
Tom Toles, The Washington Post
Gary Markstein had the same idea . . .
Gary Markstein, Milwaukee Journal Sentinel
The end of the Supreme Court's 2003 Term didn't escape the attention of our cartoonists: 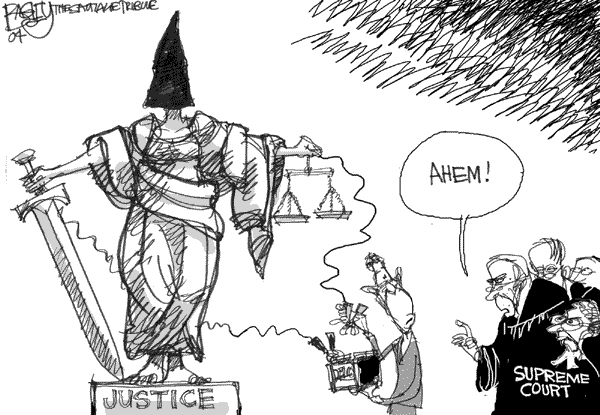
Scott Bagley, St. Lake Tribune (If this is how their covering this issue in Utah, maybe Bush really is in electoral trouble!)
Finally, the one health-care 'toon I could find from the entire month of June (I guess the Supreme Court's ERISA-pre-emption/managed care decision really was too tough to draw):
Kirk Anderson, St. Paul Pioneer Press


