
Monday, May 31, 2004
Alan Morrison . . . living greatly in the law.
Morrison's bold attempt to get Scalia out of the Cheney case is illustrative of his career:
The episode captures an essential truth about Morrison, one of the nation's top public interest lawyers, as he looks back over his 32 years with the Ralph Nader-founded Public Citizen Litigation Group. He is fearless about challenging government and corporate interests, yet also has no fear of befriending -- or at least being cordial to -- their advocates and icons.For my students, the closing words of the article are memorable:
"There's no reason not to deal with people in a civil manner," Morrison says. "I don't consider myself a rebel. I like having people return my phone calls."
Even in his parting, a range of luminaries returned his phone calls and formed a host committee for a June 3 farewell dinner for Morrison that will also fund a fellowship in his name. The list spans the spectrum of the legal establishment -- from former Whitewater Independent Counsel Kenneth Starr to Harvard Law School professor Laurence Tribe, Reagan Solicitor General Charles Fried to Clinton SG Seth Waxman, Reagan White House Counsel Fred Fielding to Clinton Chief of Staff John Podesta. "Alan Morrison is deeply respected throughout the entire Washington community," says Starr, now partner in the D.C. office of Kirkland & Ellis. "His conservative friends may not always agree with him, but they know he is a person of complete intellectual honesty."
Throughout his career, Morrison says, he was never tempted by the astronomically higher salaries he could have made if he had put his tenacity and litigating skills to work for a private firm. At Public Citizen he was paid no more than $80,000 a year, he says, and even when he supplemented that with an adjunct law school teaching gig, "I'm still making less than a first-year associate at a New York firm."
But Morrison says the rewards have been incomparable. Pointing to Craig's book about the litigation group's early days, he says, "I hope young people read it and say to themselves, 'You know, there's a better way to spend my life.' You work here, and there's no competition, you don't have to make partner." One young lawyer on his staff once told him the job was so fulfilling and fun, "I don't think we should get paid."
Morrison recalls the words of his friend the late public interest activist Joseph Rauh Jr., who once said, "They made all the money; we had all the fun."
Sunday, May 30, 2004
New Texas Supreme Court case on workers' comp.
In 1989, the Legislature enacted a new Workers’ Compensation Act in response to rising medical costs and increasing insurance premiums. The Legislature created the Texas Workers’ Compensation Commission and gave the agency broad powers to adopt rules necessary for the implementation and enforcement of the Workers’ Compensation Act. TEX. LAB. CODE § 402.061. One of TWCC’s new functions was to establish fee guidelines for reimbursements to health care providers who treat injured workers. Id. § 413.011. To this end, the agency promulgated the Texas Workers’ Compensation Commission Medical Fee Guideline 1996, adopted by reference in Rule 134.201 of the Texas Administrative Code. See 28 TEX. ADMIN. CODE § 134.201 (indicating that copies of the Guideline may be obtained from TWCC’s publication department). The Guideline contains the maximum allowable reimbursements (MARs) for thousands of medical procedures. The MARs establish upper limits on the amount of reimbursements payable to health care providers for the listed treatments or services. TWCC also promulgated a set of rules, now commonly referred to as the “Dispute and Audit Rules,” which establish a process for insurance carriers to review and audit bills submitted by health care providers. Id. §§ 133.301-.305.2 The rules also set up dispute resolution procedures to resolve disagreements over the necessity of medical procedures and the amount of reimbursements. Id. § 133.305. Requests for medical dispute resolution must be filed not later than one year from the date of the medical service. Id. § 133.305(d).The Supreme Court concludes "that TWCC complied with the statutory requisites for promulgating the fee guidelines and acted within its designated powers in limiting specified medical fee reimbursements and the time to seek medical dispute resolution. We affirm the court of appeals’ judgment on these issues. Because we conclude that TWCC did not delegate its power to private entities, we reverse the portion of the court of appeals’ judgment that is contrary to this conclusion. We also overrule the constitutional challenge to TWCC’s fee reimbursement guidelines and time limitations for commencing medical dispute resolution."
TWCC’s adoption of the Guideline and the Dispute and Audit Rules are at the base of this dispute. Patient Advocates of Texas and Allen J. Meril, M.D. (collectively PAT) initiated this lawsuit claiming that TWCC did not follow the rulemaking procedures required by statute when promulgating the Guideline. Additionally, PAT asserts that TWCC exceeded its rulemaking authority by setting a ceiling on many medical fee reimbursements and limiting the time for a party to seek medical dispute resolution to one year from the date the medical service was provided. PAT also challenges the validity of the Dispute and Audit Rules alleging that TWCC illegally delegated its audit and fee-setting authority to private insurance carriers. Lastly, PAT raises constitutional challenges to the rules on the grounds that the MARs and the one-year time limitation constitute a taking of their property without due process and just compensation. In response, TWCC argues that its enactment of the Guideline meets statutory procedural requirements and the limits placed on medical payments are consistent with the agency’s authority to establish medical policies and guidelines pursuant to section 413.011 of the Labor Code. TWCC claims that it retains its power to audit workers’ compensation participants and establish medical fees, and that the Dispute and Audit Rules are simply a means to facilitate insurance carriers’ review of the medical claims submitted by health care providers.
Radicalized elders turn to drug smuggling.
Harvard Medical amends conflict-of-interest policies
Under the policy, Harvard faculty cannot own more than $30,000 in stock from public companies that benefit from their research, a $10,000 increase from the previous limit. They cannot have any stock from companies with which they have ongoing research collaborations. In addition, faculty members cannot own stock in private companies related to their research. But faculty can receive up to $20,000 in consulting fees from companies tied to their research, also a $10,000 increase from the previous limit. . . .The Office of Public Health and Science at HHS published its own guidance on this subject on May 12.
Until now, faculty could not hold upper management jobs in firms. The new policy extends this prohibition to include the positions of chief scientific officer and chief medical officer, slots Harvard faculty occasionally accept.
The new policy was crafted by Harvard medical dean Dr. Joseph B. Martin, in consultation with several faculty committees. He noted that some faculty members argued for looser rules that would foster increased interaction with Boston's vibrant biotechnology and pharmaceutical communities.
''The issues that some individuals raised were set aside for two reasons: that we need to protect human subjects in research . . . and that there not be any perception of bias in research work," he said.
But of faculty collaborations with industry, Martin said: ''We encourage it in every possible way." The increase in the stock and consulting limits, said Martin, reflected what he thought ''responsible people ought to be able to take in."
Saturday, May 29, 2004
Cartoon roundup, Part II.

Cartoon roundup.
Jack Ohman, Portland Oregonian

Jim Morin, Miami Herald

Ed Stein, Rocky Mountain News

Randy Bish, Pittsburgh Tribune-Review
Tom Toles, Washington Post
Monkey business affects waiting times on transplant list in Albany.
Maternal-fetal conflicts: a moving target.
Interesting intersection of universal health care coverage, same-sex marriage, and domestic-partners' benefits
As pointed out in the article, however, same-sex couples may decide to remain unmarried in order to adopt children in countries than ban adoptions by married same-sex couples. And Springfield's three-month phase-out may leave out in the cold those couples that cannot (or choose not to) get their marital act together that quickly. Mayor Charlie Ryan (who was also mayor during my high school days, 37 years ago, and whom I always regarded as an exceptionally straight shooter) says his new order will bring the city into compliance with state insurance laws, though that didn't seem to be a high priority until now.
As for cost: Cambridge Vice-mayor Marjorie Decker is quoted as saying, "It’s an interesting debate for any city or town," she said. "We’re at the crossroads of what happens when you don’t have some universal form of health care." Cambridge, as well as other employers, is confronting the same choices:
Decker expects the city will eventually debate the issue. She predicts that some will argue that domestic-partnership benefits should be extended to all couples who are in long-term committed relationships, rather than forcing them to marry in order to access health benefits. . . .Stay tuned . . . .
Among Boston-area employers, Beth Israel Deaconess Medical Center and Babson College will discontinue domestic-partner benefits at the end of the year.
Employers including Massachusetts Institute of Technology, Brigham and Women’s Hospital, Massachusetts General Hospital, Fidelity Investments, Gillette Co. and EMC Corp. will maintain the benefits. Harvard University plans to maintain them for the immediate future, but will revisit the issue in the next two years.
Physician-Assisted Suicide.
In sum, the CSA was enacted to combat drug abuse. To the extent that it authorizes the federal government to make decisions regarding the practice of medicine, those decisions are delegated to the Secretary of Heath and Human Services, not to the Attorney General. The Attorney General’s unilateral attempt to regulate general medical practices historically entrusted to state lawmakers interferes with the democratic debate about physician assisted suicide and far exceeds the scope of his authority under federal law. We therefore hold that the Ashcroft Directive is invalid and may not be enforced.The irony in all this is that this most avowedly pro-State, anti-federalist administration hasn't hesitated to try to impose its will on the states when it perceived a hint of political mileage that might be gained with the far right, even when it means shamelessly trampling individual liberties and the traditional role of the states in regulating the practice of medicine.
Prisoner Abuse and Doctors' Duty.
Monday, May 17, 2004
Texas Supreme Court decides informed-consent case.
(A) Limitation of movement of shoulder and arm.This opinion is consistent with those of four Texas courts of appeals. The Supreme Court emphasized that the negligent diagnosis or prognosis could give rise to a negligence claim, but in this case the plaintiff had waived those claims over the course of the litigation. It probably doesn't need to be emphasized that the consent process may be flawed, irredeemably so, when the practitioner affirmatively misleads the patient with statements that are known to be false, simply to procure a consent.
(B) Swelling of the arm.
(C) Loss of the skin of the chest requiring skin graft.
(D) Recurrence of malignancy, if present.
(E) Decreased sensation or numbness of the inner aspect of the arm and chest wall.
None of the risks listed for this or any other procedure on List A include the risk that the physician's diagnosis or prognosis that supports his or her recommendation that the procedure be performed is or may be incorrect. If a physician told a patient that she had cancer and was therefore recommending a hysterectomy, the risks enumerated by the Texas Disclosure Panel do not include the risk that the surgery may be unnecessary. The risk that a physician may have erroneously made a diagnosis or prognosis as a predicate to recommending surgery is not inherent in any particular surgery or procedure or medication. That is a general risk of consulting a physician.
Saturday, May 08, 2004
IRS ruling a template for hospital-physician deals.
The five-page revenue ruling offers a template for how not-for-profit hospitals can protect their tax-exempt status and avoid paying unrelated-business income taxes in joint ventures with physicians or for-profit companies. . . .
[A] tax-exempt university asked for IRS guidance on its plan to offer training programs for elementary and secondary schoolteachers. The university would team up with a for-profit, interactive-video company in a 50-50 joint venture, with each partner naming three directors to the board. The governance agreement would prohibit activities contrary to the university's tax-exempt status, require the university to remain at arm's length in negotiations for contracts and other transactions, and establish fair-market value as a benchmark for prices.
The IRS said those stipulations would protect the university's tax-exempt status, and there would be no unrelated-business income taxes because the venture was an extension of the university's educational mission and insubstantial compared with its overall activity.
Friday, May 07, 2004
Schiavo timeline and significant documents.
Thursday, May 06, 2004
"Terri's Law" declared unconstitutional by Florida court.
- First, the court held that the law effects an unconstitutional delegation of legislative power to the governor, in violation of Art. II, sec. 3, of the Florida Constitution and separation-of-powers principles. The gist of this holding is that the legislature provided Gov. Bush with virtually no standards to guide his exercise of discretion as to whether to order the reinstatement of life-sustaining measures and for how long.
- Second, the court held that statute violates Terri Schiavo's right of privacy, a right that was added to the Florida Constitution in 1980 (Art. I, sec. 23). Section 23 provides: "Every natural person has the right to be let alone and free from governmental intrusion into the person's private life except as otherwise provided herein. This section shall not be construed to limit the public's right of access to public records and meetings as provided by law. "
- The court also found that the law was retroactive legislation and an unconstitutional intrusion into the judicial function.
Limits on Stem-Cell Research Re-emerge as a Political Issue.
As reported in today's New York Times, the question of the federal government's funding policies is emerging as an issue in Campaign 2004. Stay tuned . . .
Wednesday, May 05, 2004
Quality of care lacking in a majority of communities in US.
- "Profiling The Quality Of Care In Twelve Communities: Results From The CQI Study," by Eve A. Kerr, Elizabeth A. McGlynn, John Adams, Joan Keesey and Steven M. Asch.
Abstract: Health care quality falls far short of its potential nationally. Because care is delivered locally, improvement strategies should be tailored to community needs. This analysis from the Community Quality Index (CQI) study reports on a comprehensive examination of how effectively care is delivered in twelve metropolitan areas. We find room for improvement in quality overall and in dimensions of preventive, acute, and chronic care in all of these communities; no community was consistently best or worst on the various dimensions. Having concrete estimates of the extent of the gap in performance should stimulate community-based quality improvement efforts. (Full text requires subscription to journal.)
Americans get substandard care for their ailments about half the time, even if they live near a major teaching hospital, the first comprehensive study of health care provided in metropolitan areas has found.
The inadequate treatment leads to "thousands of needless deaths each year," said Dr. Elizabeth A. McGlynn, a researcher at the RAND Corporation . . . .
The study's conclusions were based chiefly on a review of the medical records of nearly 7,000 people in 12 metropolitan areas, including Newark, Miami and Orange County, Calif. On average, the authors found, patients received substandard care, as defined by leading medical groups, 50 percent to 60 percent of the time. There was little variation among the metropolitan areas, randomly selected from 60 with populations of at least 200,000. The areas included cities and their suburbs.
Texas Leads Nation in Percentage of Uninsured Workers.
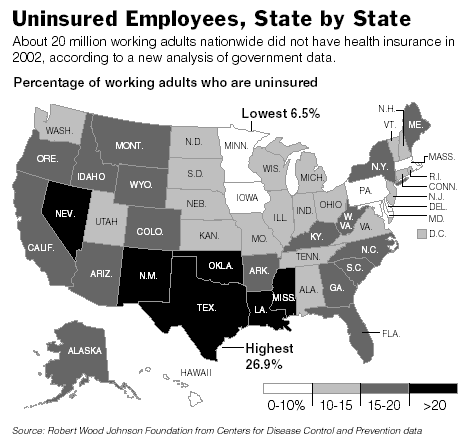
The full report (Characteristics of the Uninsured: A View from the States (May 2003), from the Robert Wood Johnson Foundation) is here.
Tuesday, May 04, 2004
Two must-read articles in the current issue of Health Affairs.
- "How Does the Quality of Care Compare in Five Countries?," by Peter S. Hussey, Gerard F. Anderson, Robin Osborn, Colin Feek, Vivienne McLaughlin, John Millar and Arnold Epstein -- 23(3):89-99.
Abstract: International data on quality of medical care allow countries to compare their performance to that of other countries. The Commonwealth Fund International Working Group on Quality Indicators collected data on twenty-one indicators that reflect medical care in Australia, Canada, New Zealand, England, and the United States. The indicators include five-year cancer relative survival rates, thirty-day case-fatality rates after acute myocardial infarction and stroke, breast cancer screening rates, and asthma mortality rates. No country scores consistently the best or worst overall. Each country has at least one area of care where it could learn from international experiences and one area where its experiences could teach others. - "U.S. Health Care Spending In An International Context," Uwe E. Reinhardt, Peter S. Hussey and Gerard F. Anderson -- 23(3):10-25.
Abstract: Using the most recent data on health spending published by the Organization for Economic Cooperation and Development (OECD), we explore reasons why U.S. health spending towers over that of other countries with much older populations. Prominent among the reasons are higher U.S. per capita gross domestic product (GDP) as well as a highly complex and fragmented payment system that weakens the demand side of the health sector and entails high administrative costs. We examine the economic burden that health spending places on the U.S. economy. We comment on attempts by U.S. policy-makers to increase the prices foreign health systems pay for U.S. prescription drugs.
HHS/CMS effort to silence CMS' chief actuary probably violated federal law.
The Congressional Research Service on Monday concluded that Bush administration officials "appear to have violated federal law" by barring CMS chief actuary Richard Foster from sharing with lawmakers his cost estimates for the Medicare legislation, the Wall Street Journal reports (Rogers, Wall Street Journal, 5/4). CRS is a branch of the Library of Congress and provides nonpartisan analysis and research to lawmakers (Pugh, Philadelphia Inquirer, 5/4). The analysis comes more than one month after Foster told members of the House Ways and Means Committee that he had shared with Doug Badger, President Bush's health policy adviser, and James Capretta, associate director of the Office of Management and Budget, his analysis that the Medicare legislation would exceed its target spending goal. According to OMB estimates released after Congress passed the legislation, the Medicare law will cost $534 billion over the next 10 years, $134 billion more than estimated by the Congressional Budget Office. Foster has said that the higher cost projection was known before the final House and Senate votes on the legislation in November but that former CMS Administrator Tom Scully told him, "We can't let that get out." In an e-mail to colleagues at CMS, Foster indicated he believed he might lose his job if he revealed his cost estimates for the Medicare legislation. Scully has said that he did not threaten to fire Foster if the higher estimates were released. Scully also said that he "curbed Foster on only one specific request" made by Democrats at the time of the first House vote on the Medicare bill (Kaiser Daily Health Policy Report, 3/25).Analysis Details. In a nine-page memo to Rep. Charles Rangel (D-N.Y.), ranking member of the Ways and Means Committee, CRS said that federal officials "do not have the right to prevent or prohibit" employees from sharing information concerning "relevant public policy issues" to congressional members (Goldstein, Washington Post, 5/4). Further, Congress' "right to receive truthful information from federal agencies to assist in its legislative functions is clear and unassailable," the analysis states. According to CRS, since 1912, federal laws have protected federal employees' rights to communicate with lawmakers, and more recent laws have "reaffirmed and strengthened" those rights (Pear, New York Times, 5/4). Jack Maskell, a legislative lawyer at CRS, said that in 1997, "when some lawmakers felt that the Clinton administration threatened the candor of federal health experts, House and Senate appropriations conferees wrote into health care legislation" that the CMS Office of the Actuary serves both the administration and the Congress, the Inquirer reports. In addition, the legislation states that the actuary's independence to provide data to Congress is "vital," according to the Inquirer (Philadelphia Inquirer, 5/4). Thus, Scully's order "would appear to violate a specific and express prohibition of federal law," according to CRS (New York Times, 5/4). However, CRS said that such an act "may not rise to level of a criminal violation" (Heil, CongressDaily, 5/3). According to the Inquirer, Scully probably could not be prosecuted because "only individual lawmakers sought Foster's estimates." Scully could not be reached for comment Monday (Philadelphia Inquirer, 5/4).
Democrats' Response. The CRS report prompted Rangel, who requested the analysis, and Rep. Pete Stark (D-Calif.), House Ways and Means Health Subcommittee ranking member, to request a new committee hearing on the estimates (CongressDaily, 5/3). According to the Journal, some House Democrats "seized the nine-page memo" to reaffirm their argument for subpoenas to make Scully and Badger testify regarding their knowledge of the "alleged 'gag order'" (Wall Street Journal, 5/4). Scully and Badger declined to appear before the House panel when it considered the estimates last month (Kaiser Daily Health Policy Report, 4/2). In a letter, Rangel and Stark reminded House Ways and Means Committee Chair Bill Thomas (R-Calif.), who has declined previous requests to subpoena Scully or Badger, that he has said he would support a subpoena "if it was clear that laws had been broken," CongressDaily reports. In the letter, Rangel and Stark said, "It is clear that laws were broken. ... Indeed, the administration's steadfast refusal even now to release the requested information raises serious questions as to the ongoing violations of the spirit, if not the letter, of these laws" (CongressDaily, 5/3). HHS Secretary Tommy Thompson last week said he would not release additional documents related to Bush administration cost estimates for the Medicare law, despite a formal request from Democrats on the House Government Reform Committee (Kaiser Daily Health Policy Report, 4/29).
Administration Reaction According to the Journal, CRS "is respected by the administration" and therefore, the CRS analysis "makes it harder to isolate the complaints as driven by election-year politics and Democrats who opposed the bill" (Wall Street Journal, 5/4). However, HHS spokesperson Bill Pierce on Monday said that the department is "focusing on instituting the new Medicare law and not on the Scully-Foster controversy" (Philadelphia Inquirer, 5/4). Pierce added that "we are looking to the future, not the past" (New York Times, 5/4).


