In sum, the CSA was enacted to combat drug abuse. To the extent that it authorizes the federal government to make decisions regarding the practice of medicine, those decisions are delegated to the Secretary of Heath and Human Services, not to the Attorney General. The Attorney General’s unilateral attempt to regulate general medical practices historically entrusted to state lawmakers interferes with the democratic debate about physician assisted suicide and far exceeds the scope of his authority under federal law. We therefore hold that the Ashcroft Directive is invalid and may not be enforced.The irony in all this is that this most avowedly pro-State, anti-federalist administration hasn't hesitated to try to impose its will on the states when it perceived a hint of political mileage that might be gained with the far right, even when it means shamelessly trampling individual liberties and the traditional role of the states in regulating the practice of medicine.
Health care law (including regulatory and compliance issues, public health law, medical ethics, and life sciences), with digressions into constitutional law, statutory interpretation, poetry, and other things that matter
Saturday, May 29, 2004
Physician-Assisted Suicide.
Prisoner Abuse and Doctors' Duty.
Monday, May 17, 2004
Texas Supreme Court decides informed-consent case.
(A) Limitation of movement of shoulder and arm.This opinion is consistent with those of four Texas courts of appeals. The Supreme Court emphasized that the negligent diagnosis or prognosis could give rise to a negligence claim, but in this case the plaintiff had waived those claims over the course of the litigation. It probably doesn't need to be emphasized that the consent process may be flawed, irredeemably so, when the practitioner affirmatively misleads the patient with statements that are known to be false, simply to procure a consent.
(B) Swelling of the arm.
(C) Loss of the skin of the chest requiring skin graft.
(D) Recurrence of malignancy, if present.
(E) Decreased sensation or numbness of the inner aspect of the arm and chest wall.
None of the risks listed for this or any other procedure on List A include the risk that the physician's diagnosis or prognosis that supports his or her recommendation that the procedure be performed is or may be incorrect. If a physician told a patient that she had cancer and was therefore recommending a hysterectomy, the risks enumerated by the Texas Disclosure Panel do not include the risk that the surgery may be unnecessary. The risk that a physician may have erroneously made a diagnosis or prognosis as a predicate to recommending surgery is not inherent in any particular surgery or procedure or medication. That is a general risk of consulting a physician.
Saturday, May 08, 2004
IRS ruling a template for hospital-physician deals.
The five-page revenue ruling offers a template for how not-for-profit hospitals can protect their tax-exempt status and avoid paying unrelated-business income taxes in joint ventures with physicians or for-profit companies. . . .
[A] tax-exempt university asked for IRS guidance on its plan to offer training programs for elementary and secondary schoolteachers. The university would team up with a for-profit, interactive-video company in a 50-50 joint venture, with each partner naming three directors to the board. The governance agreement would prohibit activities contrary to the university's tax-exempt status, require the university to remain at arm's length in negotiations for contracts and other transactions, and establish fair-market value as a benchmark for prices.
The IRS said those stipulations would protect the university's tax-exempt status, and there would be no unrelated-business income taxes because the venture was an extension of the university's educational mission and insubstantial compared with its overall activity.
Friday, May 07, 2004
Schiavo timeline and significant documents.
Thursday, May 06, 2004
"Terri's Law" declared unconstitutional by Florida court.
- First, the court held that the law effects an unconstitutional delegation of legislative power to the governor, in violation of Art. II, sec. 3, of the Florida Constitution and separation-of-powers principles. The gist of this holding is that the legislature provided Gov. Bush with virtually no standards to guide his exercise of discretion as to whether to order the reinstatement of life-sustaining measures and for how long.
- Second, the court held that statute violates Terri Schiavo's right of privacy, a right that was added to the Florida Constitution in 1980 (Art. I, sec. 23). Section 23 provides: "Every natural person has the right to be let alone and free from governmental intrusion into the person's private life except as otherwise provided herein. This section shall not be construed to limit the public's right of access to public records and meetings as provided by law. "
- The court also found that the law was retroactive legislation and an unconstitutional intrusion into the judicial function.
Limits on Stem-Cell Research Re-emerge as a Political Issue.
As reported in today's New York Times, the question of the federal government's funding policies is emerging as an issue in Campaign 2004. Stay tuned . . .
Wednesday, May 05, 2004
Quality of care lacking in a majority of communities in US.
- "Profiling The Quality Of Care In Twelve Communities: Results From The CQI Study," by Eve A. Kerr, Elizabeth A. McGlynn, John Adams, Joan Keesey and Steven M. Asch.
Abstract: Health care quality falls far short of its potential nationally. Because care is delivered locally, improvement strategies should be tailored to community needs. This analysis from the Community Quality Index (CQI) study reports on a comprehensive examination of how effectively care is delivered in twelve metropolitan areas. We find room for improvement in quality overall and in dimensions of preventive, acute, and chronic care in all of these communities; no community was consistently best or worst on the various dimensions. Having concrete estimates of the extent of the gap in performance should stimulate community-based quality improvement efforts. (Full text requires subscription to journal.)
Americans get substandard care for their ailments about half the time, even if they live near a major teaching hospital, the first comprehensive study of health care provided in metropolitan areas has found.
The inadequate treatment leads to "thousands of needless deaths each year," said Dr. Elizabeth A. McGlynn, a researcher at the RAND Corporation . . . .
The study's conclusions were based chiefly on a review of the medical records of nearly 7,000 people in 12 metropolitan areas, including Newark, Miami and Orange County, Calif. On average, the authors found, patients received substandard care, as defined by leading medical groups, 50 percent to 60 percent of the time. There was little variation among the metropolitan areas, randomly selected from 60 with populations of at least 200,000. The areas included cities and their suburbs.
Texas Leads Nation in Percentage of Uninsured Workers.
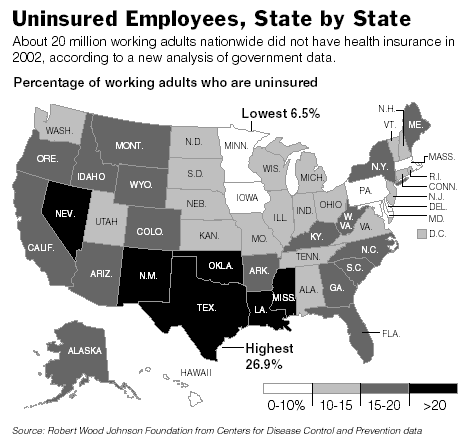
The full report (Characteristics of the Uninsured: A View from the States (May 2003), from the Robert Wood Johnson Foundation) is here.
Tuesday, May 04, 2004
Two must-read articles in the current issue of Health Affairs.
- "How Does the Quality of Care Compare in Five Countries?," by Peter S. Hussey, Gerard F. Anderson, Robin Osborn, Colin Feek, Vivienne McLaughlin, John Millar and Arnold Epstein -- 23(3):89-99.
Abstract: International data on quality of medical care allow countries to compare their performance to that of other countries. The Commonwealth Fund International Working Group on Quality Indicators collected data on twenty-one indicators that reflect medical care in Australia, Canada, New Zealand, England, and the United States. The indicators include five-year cancer relative survival rates, thirty-day case-fatality rates after acute myocardial infarction and stroke, breast cancer screening rates, and asthma mortality rates. No country scores consistently the best or worst overall. Each country has at least one area of care where it could learn from international experiences and one area where its experiences could teach others. - "U.S. Health Care Spending In An International Context," Uwe E. Reinhardt, Peter S. Hussey and Gerard F. Anderson -- 23(3):10-25.
Abstract: Using the most recent data on health spending published by the Organization for Economic Cooperation and Development (OECD), we explore reasons why U.S. health spending towers over that of other countries with much older populations. Prominent among the reasons are higher U.S. per capita gross domestic product (GDP) as well as a highly complex and fragmented payment system that weakens the demand side of the health sector and entails high administrative costs. We examine the economic burden that health spending places on the U.S. economy. We comment on attempts by U.S. policy-makers to increase the prices foreign health systems pay for U.S. prescription drugs.
HHS/CMS effort to silence CMS' chief actuary probably violated federal law.
The Congressional Research Service on Monday concluded that Bush administration officials "appear to have violated federal law" by barring CMS chief actuary Richard Foster from sharing with lawmakers his cost estimates for the Medicare legislation, the Wall Street Journal reports (Rogers, Wall Street Journal, 5/4). CRS is a branch of the Library of Congress and provides nonpartisan analysis and research to lawmakers (Pugh, Philadelphia Inquirer, 5/4). The analysis comes more than one month after Foster told members of the House Ways and Means Committee that he had shared with Doug Badger, President Bush's health policy adviser, and James Capretta, associate director of the Office of Management and Budget, his analysis that the Medicare legislation would exceed its target spending goal. According to OMB estimates released after Congress passed the legislation, the Medicare law will cost $534 billion over the next 10 years, $134 billion more than estimated by the Congressional Budget Office. Foster has said that the higher cost projection was known before the final House and Senate votes on the legislation in November but that former CMS Administrator Tom Scully told him, "We can't let that get out." In an e-mail to colleagues at CMS, Foster indicated he believed he might lose his job if he revealed his cost estimates for the Medicare legislation. Scully has said that he did not threaten to fire Foster if the higher estimates were released. Scully also said that he "curbed Foster on only one specific request" made by Democrats at the time of the first House vote on the Medicare bill (Kaiser Daily Health Policy Report, 3/25).Analysis Details. In a nine-page memo to Rep. Charles Rangel (D-N.Y.), ranking member of the Ways and Means Committee, CRS said that federal officials "do not have the right to prevent or prohibit" employees from sharing information concerning "relevant public policy issues" to congressional members (Goldstein, Washington Post, 5/4). Further, Congress' "right to receive truthful information from federal agencies to assist in its legislative functions is clear and unassailable," the analysis states. According to CRS, since 1912, federal laws have protected federal employees' rights to communicate with lawmakers, and more recent laws have "reaffirmed and strengthened" those rights (Pear, New York Times, 5/4). Jack Maskell, a legislative lawyer at CRS, said that in 1997, "when some lawmakers felt that the Clinton administration threatened the candor of federal health experts, House and Senate appropriations conferees wrote into health care legislation" that the CMS Office of the Actuary serves both the administration and the Congress, the Inquirer reports. In addition, the legislation states that the actuary's independence to provide data to Congress is "vital," according to the Inquirer (Philadelphia Inquirer, 5/4). Thus, Scully's order "would appear to violate a specific and express prohibition of federal law," according to CRS (New York Times, 5/4). However, CRS said that such an act "may not rise to level of a criminal violation" (Heil, CongressDaily, 5/3). According to the Inquirer, Scully probably could not be prosecuted because "only individual lawmakers sought Foster's estimates." Scully could not be reached for comment Monday (Philadelphia Inquirer, 5/4).
Democrats' Response. The CRS report prompted Rangel, who requested the analysis, and Rep. Pete Stark (D-Calif.), House Ways and Means Health Subcommittee ranking member, to request a new committee hearing on the estimates (CongressDaily, 5/3). According to the Journal, some House Democrats "seized the nine-page memo" to reaffirm their argument for subpoenas to make Scully and Badger testify regarding their knowledge of the "alleged 'gag order'" (Wall Street Journal, 5/4). Scully and Badger declined to appear before the House panel when it considered the estimates last month (Kaiser Daily Health Policy Report, 4/2). In a letter, Rangel and Stark reminded House Ways and Means Committee Chair Bill Thomas (R-Calif.), who has declined previous requests to subpoena Scully or Badger, that he has said he would support a subpoena "if it was clear that laws had been broken," CongressDaily reports. In the letter, Rangel and Stark said, "It is clear that laws were broken. ... Indeed, the administration's steadfast refusal even now to release the requested information raises serious questions as to the ongoing violations of the spirit, if not the letter, of these laws" (CongressDaily, 5/3). HHS Secretary Tommy Thompson last week said he would not release additional documents related to Bush administration cost estimates for the Medicare law, despite a formal request from Democrats on the House Government Reform Committee (Kaiser Daily Health Policy Report, 4/29).
Administration Reaction According to the Journal, CRS "is respected by the administration" and therefore, the CRS analysis "makes it harder to isolate the complaints as driven by election-year politics and Democrats who opposed the bill" (Wall Street Journal, 5/4). However, HHS spokesperson Bill Pierce on Monday said that the department is "focusing on instituting the new Medicare law and not on the Scully-Foster controversy" (Philadelphia Inquirer, 5/4). Pierce added that "we are looking to the future, not the past" (New York Times, 5/4).
Friday, April 30, 2004
U of Wash update.
The Seattle Times has updated its story, to reflect the actual settlement announcement this morning.
The complaint, which was filed under seal in 1999 and released today, is here.
Qui tam action against Univ. of Washington teaching hospital settles for $35 million
[W]hen [a 1996 compliance] program was put into place, auditors found rampant errors. Doctors were routinely overbilling Medicare and Medicaid, charging for more expensive services than those they had performed. According to the lawsuit, auditors found evidence of this in nine out of 10 departments at the Children's University Medical Group, the billing group for UW doctors who practice at Children's Hospital and Medical Center.Best of all, "UW Physicians destroyed the old reports, the lawsuit said, and wrote new, sanitized versions."
When UW Physicians found out, according to the lawsuit, it hid the practice by changing the compliance policy, making it acceptable to round up, meaning doctors could charge for a treatment that was one rung higher on the billing chart than the treatment they had actually provided.
With the new rules in place, UW Physicians began a second audit for 27 specialty departments. Even under the more permissive rule, though, the errors poured in, according to the lawsuit. The majority of errors came from doctors who were charging for services two or more rungs higher than the services performed. In the dermatology department, 90 percent of the cases reviewed were incorrectly billed. Rates were 57 percent for infectious-diseases, 21 percent for pulmonary and 22 percent for craniofacial.
Tuesday, April 27, 2004
Bioethics novels.
Monday, April 26, 2004
ER care being triaged at University of Colo. Hosp. in Boulder.
To begin with: "As the provider of last resort, hospital emergency departments across America have for decades accepted thousands of truly non-urgent cases and swallowed the cost. For the most part, the patients have nowhere else to go, no insurance and no money." In other words, ER patients with subacute conditions typically got triaged over to the nonemergent ER desk, where their sore throats and sprains were handled. If the bill was never paid, that was just a fact of life. No more. Now they are triaged out to another facility.
Beyond this change, the ERs are treating nonemergent ferently depending upon their financial ability to pay. Nonemergent cases will continue to be seen, as long as there's insurance coverage for that service or -- because most health plans will deny coverage of nonemergency services in the ER -- the patient has cash.
Whether this is a good thing (i.e., hospitals finally taking control of their emergency departments and running them a little more like a business) or not remains a hotly debated issue.
At least judging from the article, there is a chance that patients who present to the ER with a request for emergency services will get a cursory review, rather than a "medically appropriate screening," as required by the federal Emergency Medical Treatment and Active Labor Act (EMTALA). Federal officials say that isn't happening at the Univ. of Colo. hospital, but it is obviously a risk. And, apart from the legal liability that flows from an EMTALA violation, there is the added health costs: "'If we tell people don't come to the emergency department unless you're dying, that's exactly what they'll do,' said Arthur Kellermann, a professor at Emory University School of Medicine and chairman of the emergency medicine department at Grady Memorial Hospital in Atlanta. 'If no one else is willing to take care of that diabetic, then we are very unwise to turn that person away,' because chronic conditions tend to worsen if left untreated."
One of perhaps unintended patient benefits of EMTALA was precisely this: patients with chronic or sub-emergent conditions got seen by a doctor or nurse-practitioner/physician's assistant somewhere within the system, and conditions that could have worsened were treated sooner rather than later. The problems with this fix are (1) some ERs are stretched beyond their limits by such cases, which necessitates the diversion of true emergencies away from the ERs, and (2) from a cost standpoint, about the only more expensive (and less appropriate) hospital setting for these subacute patients is the ICU.
The message of the unsurprising story in today's paper is that our country's ER "fix" for unfunded patients (EMTALA) was an admirable attempt to fix the patient of "patient dumping" but was not a good solution -- nor was it really intended to be -- for the problem of inequitable access to health insurance, and it has become unsustainable. This was the message of a Wall Street Journal article last year about similar efforts to cut back on uncompensated care at the University of Texas Medical Branch (UTMB) at Galveston (Bernard Wysocki Jr., "At One Hospital, A Stark Solution For Allocating Care," WSJ, September 23, 2003, at A1) (may require paid subscription). In fact, the WSJ has done a good job on this issue with a series of pieces, from September to December 2003, including:
• Six Prescriptions to Ease Rationing, 12/22/03Meanwhile, a quite useful analysis of the "hidden costs" in the Canadian health care system appeared last week in the WSJ and should be required reading for anyone who thinks health-care financing woes are subject to a quick fix.
• Universal Care Has a Big Price: Patients Wait, 11/12/03
• Longer Dialysis Raises Hopes, but Poses Dilemma, 10/02/03
• Stark Choices at a Texas Hospital, 09/23/03
• Lilly Fuels Debate Over Rationing, 09/18/03
• An Invisible Web of Gatekeepers, 09/16/03
• Health Care's Big Secret: Rationing Is Here, 09/12/03
Sunday, April 25, 2004
The New York Times: "Administration Says a `Zone of Autonomy' Justifies Its Secrecy on Energy Task Force"
Do poets die young(er)?
"Poets produce twice as much of their lifetime output in their twenties as novelists do," he said.Good. Now we can go back to worrying about real health threats, like SARS and the environmental policies of George Bush.
So when a budding novelist dies young, few people may notice.
"A great novelist or nonfiction writer who dies at 28 may not have yet produced her or his magnum opus."
Kaufman said poets should not worry, but should perhaps look after their health.
"The fact that a Sylvia Plath ... may die young does not necessarily mean an Introduction to Poetry class should carry a warning that poems may be hazardous to one's health," he said.
Gov. Romney won't let gay outsiders wed in Massachusetts.
It seems unlikely that any state would be able to say that at the moment. Thirty-nine states have passed so-called defense-of-marriage acts, which stipulate that marriage is between a man and a woman. Three other states — Maryland, New Hampshire and Wyoming — have laws precluding same-sex marriage. And seven states, including New York, New Jersey and Connecticut, make no specific reference to same-sex couples in their laws.By my count, that's 49 states that will not recognize same-sex marriage. (Where's D.C. in all this?)
Described by various news reports as "obscure" and "little-known," the 1913 law is easily found in Chapter 207 ("Marriage") of the Domestic Relations Law of the Commonwealth of Massachusetts. The first part of Chapter 207 is entitled "Certain Marriages Prohibited," and Section 11 (of 14 sections) lays it out for all to see:
Section 11. No marriage shall be contracted in this commonwealth by a party residing and intending to continue to reside in another jurisdiction if such marriage would be void if contracted in such other jurisdiction, and every marriage contracted in this commonwealth in violation hereof shall be null and void.I don't know of many other states with a similar provision, probably because most states are happy to marry 'most anyone who meets the legal requirements of their own state and leave it to the happy couples' home states to figure out whether they will recognize the union or not (depending on whether the marriage violates the public policy of the state). Gov. Romney, on the other hand, is not concerned with enforcing other states' rules about who can marry whom. His worry is that Massachusetts will "become the Las Vegas of same-sex marriage." Considering that all states are perfectly capable of protecting their own interests in traditional marriage without the help of the Commonwealth of Massachusetts, one wonders whether this is really about the proliferation of tacky little white marriage chapels or plain, old-fashioned discrimination.
Saturday, April 24, 2004
More medical hoax sites on the WWW
Wednesday, April 21, 2004
Google Search: cloning
Godsend Institute.
Sunday, April 18, 2004
Infectious disease . . . and the duty to treat: what are the limits?
Wednesday, April 14, 2004
Health care and IT.
So why has health care almost uniquely failed to invest in IT? First, the industry remains fragmented, with few entities big enough to make the necessary sizable upfront investment. Even in cases where hospitals or doctors' practices might be large enough, the economic incentives are pretty weak. In an industry in which service providers are still paid largely on the basis of how much they do, investing in systems that would help reduce the number of tests and procedures isn't the most obvious way to boost incomes.All of this raises an obvious question: what can the government do, through Medicare conditions of participation and through changes in reimbursement, to encourage the transition to a safer and more efficient system?
The networked quality of the health care industry, with independent doctors, hospitals, labs and pharmacies all providing services to the same patient, also discourages IT investment. Any economic gains wouldn't be fully captured by the entity making the investment, but would be likely to leak out to other providers or the insurer. And because the big payoff from such investments comes only after lots of other enterprises install the same system and make it possible for information to be easily shared, there's little incentive to be first.
Finally, there are the doctors, who still pretty much control the health care system and, up to now, have resisted anything that threatens to increase their workload, change the way they practice or limit their medical discretion. It is no coincidence that some of the earliest successes have come at Veterans Affairs hospitals, where doctors are salaried employees.
Saturday, April 10, 2004
HR 3108 signed into law
April 10, 2004
STATEMENT BY THE PRESS SECRETARY
H.R. 3108, the 'Pension Funding Equity Act of 2004,' which establishes a two-year temporary replacement of the benchmark interest rate for determining funding liabilities of private sector pension plans; establishes temporary alternative minimum funding requirements for certain underfunded pension plans; and allows certain multiemployer plans to temporarily delay the amortization of specified losses.
Parkland's not the only one . . . .
The new "financial improvement committee" will give county officials greater power and control over the beleaguered medical center, which was once operated by the county but was spun off into a public-benefit corporation in 1997. Though Westchester has little direct control over the hospital corporation, the county is ultimately liable for its debts.
After the corporation posted two straight years of deficits totaling nearly $140 million, Westchester officials told hospital officials to set up the oversight committee or risk losing county financing.
"It gives us an ability to watch what goes on," said Bill Ryan, the chairman of the Westchester Legislature and a member of the committee. "We can't accept business as usual. There's been a tremendous failure over there over six years."
More on the pension bill that may kill the antitrust challenge to The Match.
First, the legislation contains an explicit exception stating that it does not apply to price-fixing claims and Judge Paul Friedman noted in a recent ruling that plaintiffs have brought such a claim. Senators Bingaman and Feingold both noted on the Senate floor that the legislation does not apply to the residents' lawsuit.Despite the experience of Sen. Bingaman's wife, Anne, in heading the Antitrust Division of DOJ during the Clinton Administration, Bingaman's and Feingold's comments may not amount to much, considering their opposition to the inclusion of this provision in the pension bill. But they do have a point . . . .
Second, any legislation depriving tens of thousands of medical residents of the same antitrust protections enjoyed by all other Americans would be unconstitutional. At stake are not only the constitutional rights of medical residents, but the rights of workers in all other industries where employers have the political clout to force unfair wages through price-fixing and cover it up with secretive, insulating legislation.
In the district court's opinion (undated, but handed down Feb. 21, 2004), the court noted (beginning at p. 60) that "Plaintiffs raise one claim of price-fixing against all defendants under Section 1 of the Sherman Act." In response to motions to dismiss under Fed. R. Civ. P. 12(b)(6) for failure to state a claim, the court wrote that it "concludes that plaintiffs adequately have alleged a common agreement to displace competition in the recruitment, hiring, employment and compensation of resident physicians and to impose a scheme of restraints, which have the purpose and effect of fixing, artificially depressing, standardizing and stabilizing resident physician compensation and other terms of employment among a number of the named organizational defendants and those institutional defendants that participated in the Match Program."
Friday, April 09, 2004
Antitrust challenge to the residency match may be about to bite the dust.
The law was passed by Congress on Thursday. The last-minute provision -- Section 207 -- exempts residency matching programs and sponsors from antitrust laws, other than for price-fixing claims:
SEC. 207. CONFIRMATION OF ANTITRUST STATUS OF GRADUATE MEDICAL RESIDENT MATCHING PROGRAMS.
(a) FINDINGS AND PURPOSES-
(1) FINDINGS- Congress makes the following findings:
(A) For over 50 years, most United States medical school seniors and the large majority of graduate medical education programs (popularly known as `residency programs') have chosen to use a matching program to match medical students with residency programs to which they have applied. These matching programs have been an integral part of an educational system that has produced the finest physicians and medical researchers in the world.
(B) Before such matching programs were instituted, medical students often felt pressure, at an unreasonably early stage of their medical education, to seek admission to, and accept offers from, residency programs. As a result, medical students often made binding commitments before they were in a position to make an informed decision about a medical specialty or a residency program and before residency programs could make an informed assessment of students' qualifications. This situation was inefficient, chaotic, and unfair and it often led to placements that did not serve the interests of either medical students or residency programs.
(C) The original matching program, now operated by the independent non-profit National Resident Matching Program and popularly known as `the Match', was developed and implemented more than 50 years ago in response to widespread student complaints about the prior process. This Program includes on its board of directors individuals nominated by medical student organizations as well as by major medical education and hospital associations.
(D) The Match uses a computerized mathematical algorithm, as students had recommended, to analyze the preferences of students and residency programs and match students with their highest preferences from among the available positions in residency programs that listed them. Students thus obtain a residency position in the most highly ranked program on their list that has ranked them sufficiently high among its preferences. Each year, about 85 percent of participating United States medical students secure a place in one of their top 3 residency program choices.
(E) Antitrust lawsuits challenging the matching process, regardless of their merit or lack thereof, have the potential to undermine this highly efficient, pro-competitive, and long-standing process. The costs of defending such litigation would divert the scarce resources of our country's teaching hospitals and medical schools from their crucial missions of patient care, physician training, and medical research. In addition, such costs may lead to abandonment of the matching process, which has effectively served the interests of medical students, teaching hospitals, and patients for over half a century.
(2) PURPOSES- It is the purpose of this section to--
(A) confirm that the antitrust laws do not prohibit sponsoring, conducting, or participating in a graduate medical education residency matching program, or agreeing to do so; and
(B) ensure that those who sponsor, conduct or participate in such matching programs are not subjected to the burden and expense of defending against litigation that challenges such matching programs under the antitrust laws.
(b) APPLICATION OF ANTITRUST LAWS TO GRADUATE MEDICAL EDUCATION RESIDENCY MATCHING PROGRAMS-
(1) DEFINITIONS- In this subsection:
(A) ANTITRUST LAWS- The term `antitrust laws'--
(i) has the meaning given such term in subsection (a) of the first section of the Clayton Act (15 U.S.C. 12(a)), except that such term includes section 5 of the Federal Trade Commission Act (15 U.S.C. 45) to the extent such section 5 applies to unfair methods of competition; and
(ii) includes any State law similar to the laws referred to in clause (i).
(B) GRADUATE MEDICAL EDUCATION PROGRAM- The term `graduate medical education program' means--
(i) a residency program for the medical education and training of individuals following graduation from medical school;
(ii) a program, known as a specialty or subspecialty fellowship program, that provides more advanced training; and
(iii) an institution or organization that operates, sponsors or participates in such a program.
(C) GRADUATE MEDICAL EDUCATION RESIDENCY MATCHING PROGRAM- The term `graduate medical education residency matching program' means a program (such as those conducted by the National Resident Matching Program) that, in connection with the admission of students to graduate medical education programs, uses an algorithm and matching rules to match students in accordance with the preferences of students and the preferences of graduate medical education programs.
(D) STUDENT- The term `student' means any individual who seeks to be admitted to a graduate medical education program.
(2) CONFIRMATION OF ANTITRUST STATUS- It shall not be unlawful under the antitrust laws to sponsor, conduct, or participate in a graduate medical education residency matching program, or to agree to sponsor, conduct, or participate in such a program. Evidence of any of the conduct described in the preceding sentence shall not be admissible in Federal court to support any claim or action alleging a violation of the antitrust laws.
(3) APPLICABILITY- Nothing in this section shall be construed to exempt from the antitrust laws any agreement on the part of 2 or more graduate medical education programs to fix the amount of the stipend or other benefits received by students participating in such programs.
(c) EFFECTIVE DATE- This section shall take effect on the date of enactment of this Act, shall apply to conduct whether it occurs prior to, on, or after such date of enactment, and shall apply to all judicial and administrative actions or other proceedings pending on such date of enactment.
More on drugs: Reimportation.
Legalizes reimportation (or importation) of prescription drugs from FDA approved exporters. To be approved, registered exporters must agree to meet safety requirements and to permit FDA inspectors on their premises full time to ensure compliance.
Overview of Key Elements of the REMEDIES Act of 2004
Creates a "fast-track" regulatory process for FDA to implement the importation system quickly.
Importation of qualified prescription drugs from Canada is immediately legalized while the new importation system is developed and implemented by FDA.
Under the new system, individuals, pharmacies, and drug wholesalers are permitted to legally import prescription drugs from registered foreign exporters:o Individuals may order drugs from a registered exporter pursuant to a valid prescription issued by a U.S. doctor and filled by a pharmacist whose licensing requirements are equivalent to those required in the U.S. or by a dispensing pharmacist duly licensed by a state.Drugs imported to U.S. pharmacies and drug wholesalers must be FDA approved drugs produced in the United States or in FDA inspected manufacturing facilities in other counties. FDA is required to provide the proper labeling for drugs for importation.
o Commercial shipments are permitted only to licensed pharmacists for resale directly to consumers and by drug wholesalers who can sell to pharmacies as they do today.
The FDA through its inspectors is responsible for tracing all drugs exported to the US back to their original manufacturing plant and ensuring that they have been stored and transported safely from that plant.
Individuals may also purchase drugs that are bioequivalent to FDA-approved brand name drugs that are produced by the same brand-name manufacturer.o These drugs are drugs not technically approved by the FDA but the foreign government has approved the drug and that drug has the same active ingredient or ingredients as the FDA-approved drug and the same route of administration, dosage form, and strength.A User Fee charged to registered exporters provides the financing to provide the resources to FDA to ensure the safety of imported drugs.
o If a drug manufacturer believes, however, that the non-FDA approved drug is not bioequivalent to the FDA approved drug, then it must submit a petition to the FDA to show that (a) the differences result in a product that is not bioequivalent to the drug approved in the U.S., and (b) that such differences are due to scientifically and legally valid differences in the regulatory requirements of the U.S. and the country(ies) in which the apparently similar drug is marketed. The manufacturer is required to pay a user fee sufficient to cover the cost of the FDA's review of the petition and supporting documentation.o User fees charged to registered exporters would be sufficient to cover all costs including those incurred for inspection and verification within the United States, at the exporter's premises and any other location where the drugs have been stored prior to entry into the U.S.
o The FDA would be required to verify the source and inspect the intermediate handlers of all drugs intended for export into the United States.
o FDA would also be required to determine by a statistically significant sample that the recipients held valid prescriptions (individuals ordering 90-day supply or less) or verify that recipient was a licensed pharmacy that only dispensed drugs to individuals.
The FDA would also be required to supply valid U.S. labeling upon request of the registered exporter and affix or supervise the affixing of seals, markings or tracking technology that would inform border personnel that such imports were lawful to be entered as labeled.
Drugs not permitted for importation include controlled substances and certain other drugs not appropriate for importation because of storage, significant safety concerns, or drugs that are more likely to be counterfeited.
Provisions to Protect Safety of the Public:
Unauthorized imports would be treated as contraband and would be seized and destroyed upon entry without notice.
For the first two years, importation would be limited to Canada. The Department of Health and Human Services would submit a report to Congress in the second year, and unless Congress changed the law, countries from which importation is permitted would be expanded to include, the European Union, the European Free Trade Association, Japan, Australia, and New Zealand. Other countries meeting statutory criteria could also be added to the list by the Secretary.
The legislation continues to prohibit the import or reimport of drugs supplied free or at nominal cost to charitable or humanitarian organizations including the United Nations or a government of a foreign country.
Requires pedigrees from the manufacturer to the dispensing pharmacist for all prescription drugs sold within the U.S. or to an exporter authorized to export drugs into the U.S.
Requires the automatic suspension of an exporter's registration for any attempted entry of non-qualified or unsafe drugs with restricted ability to seek re-instatement in the future.
Requires that registered exporters submit to the jurisdiction of the U.S. federal court system and provides a mechanism for civil actions against the property of persons that import non-qualified drugs.
Repeals the provision in the Controlled Substances Act that permits the personal import of scheduled drugs, which is a significant source of illegal drug trade in the U.S. Tax Incentives for Manufacturers to Facilitate Reimportation
Incentive To Not Prevent Reimportation: Manufacturers that do not take any action, directly or indirectly, to prevent reimportation receive a 20% increase in R&D tax credit for that year.
Penalty For Preventing Reimportation: Manufacturers that take any action, directly or indirectly, to prevent authorized reimportation lose the business expense deduction for advertising expenses.
Thursday, April 08, 2004
Drug costs redux.
The Medicare reform law last fall [Pub. L. No. 108-173] falls into that latter category: many Medicare beneficiaries will pay more out of pocket for their drugs than before this so-called reform, and their ability to lay off the risk through third-party insurance is restricted by the law. But the political message was, "Hi, we're Congress and we're here to help you with your staggering drug bills," and AARP and others bought it. (Tip: When the drug companies support a drug reform bill, hold on to your wallet.)
Maine has been experimenting with a plan to keep drug costs low for Medicaid beneficiaries, and despite being fought tooth and nail by the drug companies' representative, they had their law upheld in the Supreme Court last Term [PhRMA v. Walsh].
In addition, the on-going controversy over reimportation of drugs from Canada is a symptom of the lengths to which employers will go in order to lower sky-high drug costs, as well as the absurd lengths to which the FDA will sometimes go to promote the interests of Big Pharm. (Thankfully, this policy is currently under review, though nothing is expected to come of the review anytime soon.)
More recently, the Detroit Free Press reports in yesterday's paper that Michigan's drug price control law was upheld by the D.C. Circuit last week. The case, PhRMA v. Thompson, No. 02-5117 (D.C. Cir. April 2, 2004), affirmed summary judgment for DHHS, which had been sued by PhRMA for approving the Michigan plan ("the Initiative")"
Under the Initiative, if a drug manufacturer does not sign each of two specified rebate agreements with Michigan—one to provide rebates for drugs the state purchases for Medicaid recipients and the other to provide identical rebates for drugs the state purchases for the two non-Medicaid state health programs—the drug will be covered under the programs subject to ‘‘prior authorization.’’. . .The court concluded that the resulting plan adequately promotes the best interests of patients and provides for a suitable appeal mechanism is a physician believes a nonlisted drug would be better for the patient than one of the discounted listed drugs.
Wednesday, April 07, 2004
Been down so long, it looks like up to me.
Since I've been gone:
For example, the OIG recently alleged that a physician violated his assignment agreement when he presented to his patients -- including Medicare beneficiaries – a “Personal Health Care Medical Care Contract” asking patients to pay an annual fee of $600. While the physician characterized the services to be provided under the contract as “not covered” by Medicare, the OIG alleged that at least some of these contracted services were already covered and reimbursable by Medicare. Among other services offered under this contract were the “coordination of care with other providers,” “a comprehensive assessment and plan for optimum health,” and “extra time” spent on patient care. OIG alleged that based on the specific facts and circumstances of this case, at least some of these contracted services were already covered and reimbursable by Medicare. Therefore, OIG alleged that each contract presented to this physician’s Medicare patients constituted a request for payment for already covered services, other than the coinsurance and deductible, and was therefore a violation of the physician’s assignment agreement.As I read it, this was a somewhat inept attempt to create a "boutique" or "concierge" practice with Medicare patients -- a topic I've addressed before, here and here.
Sunday, March 28, 2004
Medicare: belly up or double down?
The trustees' report does, however, give one more reason to hate the prescription drug bill the administration rammed through Congress last year. If deception, intimidation, abuse of power and giveaways to drug companies aren't enough, it turns out that the bill also squanders taxpayer money on H.M.O.'s. . . .I hate to say 'I told you so,' but the consistent line from this blogger since last July has been that the Rx benefit was too expensive and not a sufficient benefit to those who need the help with their medications. Subsequent analysis and news have borne this out: (1) the true cost of the bill was intentionally underestimated by 25 percent and (2) the true beneficiaries of the bill are the pharmaceutical companies and the HMOs.
But whether because of ideology or because of H.M.O. campaign contributions, the people now running the country refuse to learn that lesson. As part of last year's prescription drug bill, they tried again, offering an even bigger subsidy to private plans.
And that turns out to be an important reason for the deterioration in Medicare's prospects: of the seven years lopped off the life of the trust fund, two are the result of increased subsidies mandated by last year's law, mainly in the form of higher payments to H.M.O.'s.
So what did we learn this week? Social Security is in decent shape. Medicare has problems, but ill-conceived "reform" has only made those problems worse. And let's rip up that awful prescription drug bill and start over.
When (and how) will the Administration's chicaneries catch up to Dubya? Time will tell . . . . Speaking of chicaneries, check out this report: "United States House of Representatives, Committee on Government Reform -- Minority Staff Special Investigations Division (March 16, 2004): Iraq on the Record -- The Bush Administration's Public Statements on Iraq, prepared for Rep. Henry A. Waxman."
Saturday, March 27, 2004
Seventh Circuit Court of Appeals Backs Privacy of Hospital Abortion Records
He based this ruling on their sensitivity, which he compared to that of psychotherapists’ treatment records, held privileged in Jaffee v. Redmond, 518 U.S. 1 (1996). The creation of new common law evidentiary privileges is authorized by Fed. R. Evid. 501, and Jaffee is not the only recent case in which the authority was exercised. Goodyear Tire & Rubber Co. v. Chiles Power Supply, Inc., 332 F.3d 976, 979–81 (6th Cir. 2003); In re Air Crash Near Cali, Colombia, 959 F. Supp. 1529, 1533–35 (S.D. Fla. 1997), and United States v. Lowe, 948 F. Supp. 97, 99–100 (D. Mass. 1996), all created new privileges on the authority of Jaffee. But none relates to medical records and we are reluctant to embark on a case-by-case determination of the relative sensitivity of medical records of different ailments or procedures. Most medical records are sensitive, and many are as sensitive as late-term abortion records, such as the records of AIDS patients. Proceeding down the path taken by the district court would inevitably result in either arbitrary line drawing or the creation of an Illinois-type comprehensive privilege for medical records. Northwestern Memorial Hospital concedes that there is no federal common law physician-patient privilege. It is not for us—especially in so summary a proceeding as this litigation to quash the government’s subpoena—to create one, whether all at once or by a process of slow but inevitable additions to the sole category recognized by Jaffee.The government wants abortion records on patients of doctors who are challenging the constitutionality of the Partial-Birth Abortion Ban Act of 2003, Pub. L. No. 108–105, 117 Stat. 1201, 18 U.S.C. § 1531, presumably to impeach them when they testify as medical experts in their own case. The Court of Appeals ultimately decided the burdens of production outweighed the benefits to the government:
What is true is that the administrative hardship of compliance would be modest. But it is not the only or the main hardship. The natural sensitivity that people feel about the disclosure of their medical records—the sensitivity that lies behind HIPAA—is amplified when the records are of a procedure that Congress has now declared to be a crime. Even if all the women whose records the government seeks know what “redacted” means, they are bound to be skeptical that redaction will conceal their identity from the world.The full opinion contains the suggestion -- hinted at rather than explicitly stated -- that the government's true motivation in seeking these records from hospitals across the country is harrassment -- of physicians and patients alike. The Court of Appeals may not have created a new common-law privilege, but it did the next best thing.
This is hardly a typical case in which medical records get drawn into a lawsuit. Reflecting the fierce emotions that thelong-running controversy over the morality and legality of abortion has made combustible, the Partial-Birth Abortion Ban Act and the litigation challenging its constitutionality—and even more so the rash of suits around the country in which the Department of Justice has been seeking the hospital records of abortion patients—have generated enormous publicity. These women must know that, and doubtless they are also aware that hostility to abortion has at times erupted into violence, including criminal obstruction of entry into abortion clinics, the firebombing of clinics, and the assassination of physicians who perform abortions. Some of these women will be afraid that when their redacted records are made a part of the trial record in New York, persons of their acquaintance, or skillful “Googlers,” sifting the information contained in the medical records concerning each patient’s medical and sex history, will put two and two together, “out” the 45 women, and thereby expose them to threats, humiliation, and obloquy. . . .
Even if there were no possibility that a patient’s identity might be learned from a redacted medical record, there would be an invasion of privacy. Imagine if nude pictures of a woman, uploaded to the Internet without her consent though without identifying her by name, were downloaded in a foreign country by people who will never meet her. She would still feel that her privacy had been invaded. The revelation of the intimate details contained in the record of a late-term abortion may inflict a similar wound.
If Northwestern Memorial Hospital cannot shield its abortion patients’ records from disclosure in judicial proceedings, moreover, the hospital will lose the confidence of its patients, and persons with sensitive medical conditions may be inclined to turn elsewhere for medical treatment. It is not as if the government were seeking medical records from every hospital and clinic that performs late-term abortions, in which event women wanting assurance against the disclosure of their records would have nowhere to turn. It is Dr. Hammond’s presence in the New York suit as plaintiff and expert that has resulted in the government’s subpoenaing Northwestern Memorial Hospital. . . .
The merits of the dispute are for determination at trial. The only issue for us is whether, given that there is a potential psychological cost to the hospital’s patients, and a potential lost in lost goodwill to the hospital itself, from the involuntary production of the medical records even as redacted, the cost is offset by the probative value of the records. The district judge presiding at the trial has said that the records are “relevant,” and no doubt they are—in the attenuated sense in which nonprivileged materials may be sought in discovery. “Relevant information need not be admissible at the trial if the discovery appears reasonably calculated to lead to the discovery of admissible evidence.” Fed. R. Civ. P. 26(b)(1); see Oppenheimer Fund, Inc. v. Sanders, 437 U.S. 340, 350–52 (1978); CSC Holdings, Inc. v. Redisi, 309 F.3d 988, 995–96 (7th Cir. 2002). The trial judge has not opined on the probative value of the records, which appears to be meager. . . .
The Partial-Birth Abortion Ban Act was passed, as we said, in response to the Supreme Court’s decision in the Stenberg case. Stenberg was one of a number of “first generation” partial-birth cases. . . .
Were the government sincerely interested in whether D & X abortions are ever medically indicated, one would have expected it to seek from Northwestern Memorial Hospital statistics summarizing the hospital’s experience with late-term abortions. Suppose the patients who undergo D & X abortions are identical in all material respects (age, health, number of weeks pregnant, and so on) to those who undergo procedures not forbidden by the Partial-Birth Abortion Ban Act. That would be potent evidence that the D & X procedure does not have a compelling health rationale. No such evidence has been sought, in contrast to the Planned Parenthood case, supra, at Transcript 26 (Mar. 5, 2004). A variant of the suggested approach would be to obtain a random sample of late-term abortion records from various sources and then determine, through good statistical analysis, whether the patient characteristics that lead Dr. Hammond to perform a D & X lead other physicians to perform a conventional D & E instead, and whether there are differences in the health consequences for these two groups of women. If there are no differences, the government might have a good defense of the Act. Gathering records from Hammond’s patients alone will not be useful; but if the government has other records (say, from VA hospitals) already in its files, then records of Hammond’s procedures might enable a useful comparison. The government hasn’t suggested doing anything like that either. Its motives in seeking individuals’ medical records remain thoroughly obscure.
The question whether the D & X procedure is ever medically indicated will be resolved as a matter of legislative fact not requiring the taking of trial-type testimony at all (see Hope Clinic v. Ryan, supra, 195 F.3d at 885 (dissenting opinion)), or will pivot on the clash of expert witnesses at the New York trial, or perhaps, as suggested in Stenberg, will be answered by some combination of these two approaches to determining facts. The medical records of expert witnesses are irrelevant to the first inquiry; and, so far as we can determine after having listened to the government’s arguments at length, those records will not figure significantly in the resolution of experts’ disagreements either.
The fact that quashing the subpoena comports with Illinois’ medical-records privilege is a final factor in favor of the district order’s action. As we held in Memorial Hospital for McHenry County v. Shadur, 664 F.2d 1058, 1061 (7th Cir. 1981), comity “impels federal courts to recognize state privileges where this can be accomplished at no substantial cost to federal substantive and procedural policy.” See also United States v. One Parcel of Property Located at 31–33 York Street, 930 F.2d 139, 141 (2d Cir. 1991) (per curiam). Patients, physicians, and hospitals in Illinois rely on Illinois’ strong policy of privacy of medical records. They cannot rely completely, for they are not entitled to count on the state privilege’s being applied in federal court. But in a case such as this in which, so far as we can determine, applying the privilege would not interfere significantly with federal proceedings, comity has required us not to apply the Illinois privilege, but to consider with special care the arguments for quashing the subpoena on the basis of relative hardship under Fed. R. Civ. P. 45(c).
Tuesday, March 23, 2004
SCOTUS heard oral arguments in the Texas HMO case today.
Sunday, March 21, 2004
One Crucial Issue in Pledge Case: What Does "Under God" Mean?
Law profs weigh in on Scalia's recusal decision.
Saturday, March 20, 2004
More on Scalia's recusal refusal.
It would have been better for us all, especially for Scalia and the Court, if he hadn't gone duck hunting with the Veep three weeks after the Court granted cert. in Cheney's case. If Scalia truly believes in his heart that this is not true, he has as tin an ear for appearances as he has been accused of having. But it did happen. And he explained it in as direct and forceful a manner as one could wish. Is there still an appearance of impropriety? Do you really believe that Scalia has left the impression that he might throw the case for Cheney?
A generation ago, conservatives mounted a witch hunt to get William O. Douglas off the Court. Liberals howled, even though Douglas probably gave his opponents more impeachment fodder to work with than Scalia ever will. Going after the scalps of justices whose positions we oppose may seem like sport, but it can be turned against justices whose positions we support in a heartbeat. And, regardless of whose ox is getting gored, the Court and the rest of us are the losers at the end of this game.
CMS issues guidance for exceptions to specialty-hospital moratorium.
For most transactional lawyers, the guidance probably comes a little too late to do their clients any real good, since the race was on last fall to get specialty hospitals "grandfathered" before the moratorium took effect on November 18, 2003. According to the press release, "The MMA also excludes from the moratorium (or grandfathers), hospitals that were in operation before or under development as of November 18, 2003. In determining whether a hospital was under development as of that date, the law instructs CMS to consider whether architectural plans were completed, funding was received, zoning requirements were met, and necessary approvals from appropriate State agencies were received. CMS can also consider additional evidence that would indicate whether a specialty hospital was under development."
Bush Medicare Reform Bill Become a Nightmare for GOP.
But less than four months after he signed it into law on Dec. 8, Bush's Medicare-reform dream has turned into a nightmare and a potential drag on his bid for re-election.You can say that again. In fact, the NY Times did, in today's editorial: "Credibility is indeed at the heart of the matter — not only for the media, but also for an administration intent on spinning its way toward November."
-- The Bush administration deliberately didn't tell Congress that the measure could cost more than $100 billion more than advertised.
-- House Republican leaders abused House rules to push the measure to a narrow victory. There are also allegations of threats and bribes that are under investigation.
-- The Bush administration spent millions of taxpayer dollars on public service TV ads touting the Medicare reform law that look suspiciously like Bush campaign commercials. Those, too, are now under investigation.
-- Polls show that a majority of Americans don't like the Medicare reforms.
"It's something that's eating away at the credibility of the administration in an election year on a bill that he (Bush) thought was a building block for his re-election," said Stephen Hess, a political analyst for the Brookings Institution, a centrist think tank, and a former aide to President Eisenhower.
Times editorial on administration's phony TV ads.
Medicare Actuary Gives Wanted Data to Congress.
Friday, March 19, 2004
"F"-word illegal on broadcast airwaves (can cable be far behind?) . . .
WARNING: Mature Content Follows. Read at your own risk.The FCC handed down its decision Thursday in the case of the NBC stations' broadcast of the Golden Globes Awards in January 2003, at which Bono (lead singer for U2) accepted his award with the immortal words, "This is really, really, fucking brilliant. Really, really great." The Enforcement Bureau dismissed the numerous complaints filed against this broadcast because, the Bureau reasoned, "the material was not obscene or indecent, finding in particular with respect to indecency that the language used by Bono did not describe, in context, sexual or excretory organs or activities and that the utterance was fleeting and isolated." The FCC reversed, holding that fuck is always indecent or obscene:
"use of the phrase at issue is within the scope of our indecency definition because it does depict or describe sexual activities. We recognize NBC’s argument that the 'FWord' here was used 'as an intensifier.' Nevertheless, we believe that, given the core meaning of the 'F-Word,' any use of that word or a variation, in any context, inherently has a sexual connotation, and therefore falls within the first prong of our indecency definition. This conclusion is consistent with the Commission’s original Pacifica decision, affirmed by the Supreme Court, in which the Commission held that the 'F-Word' does depict or describe sexual activities."The Commission also found fuck to be profane:
We also find, as an independent ground, that the use of the phrase at issue here in the context and at the time of day here constitutes “profane” language under 18 U.S.C. § 1464. The term “profanity” is commonly defined as “vulgar, irreverent, or coarse language.”34 The Seventh Circuit, in its most recent decision defining “profane” under section 1464, stated that the term is “construable as denoting certain of those personally reviling epithets naturally tending to provoke violent resentment or denoting language so grossly offensive to members of the public who actually hear it as to amount to a nuisance.”35 We find that the broadcast of the phrase at issue here in the context and at the time of day qualifies as “profane” under the Seventh Circuit nuisance rationale.36 Use of the “F-Word” in the context at issue here is also clearly the kind of vulgar and coarse language that is commonly understood to fall within the definition of “profanity.”Can this definition of "profane" can survive First Amendment scrutiny?
I have a hard time arguing that Bono should be able to say fuck whenever and wherever he wants. It doesn't bother me terribly that the FCC wants to keep the broadcast airwaves free of such crudity. But the FCC's reasoning is off: the Enforcement Bureau was right to focus on the lack of any sexual context and the fleeting, inadvertent, unscripted nature of Bono's use. And where does this end? With 7-second delays on all football games and tennis matches so that the excited utterances of linemen and McEnroe wannabe's will be cleansed from our ears? For the time being, cable is exempt from this ruling, but there appears to be no reason in law why cable franchises can't be held to the same standard as broadcasters. That would seem to be the message from the Court in Denver Area Educational Telecommunications Consortium, Inc., et al. v. Federal Communications Commission et al. ("Cable television broadcasting, including access channel broadcasting, is as 'ccessible to children' as over the air broadcasting, if not more so. Cable television systems, including access channels, 'have established a uniquely pervasive presence in the lives of all Americans.' 'Patently offensive' material from these stations can 'confron[t] the citizen' in the 'privacy of the home with little or no prior warning.'")
Cleaning up the airwaves so that those who want to avoid crude speech and images is a fine goal. But it should be done in a manner that does not throw out the baby with the bathwater. The FCC's opinion in this case has no limiting principal and no handrail for the slippery slope to censorship of all artistic expression (yes, including the artistic use of fuck).